
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001816 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001816

|
|||||||
Regist Date |
2012/06/21 18:02:55 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
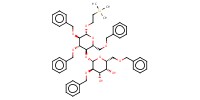
Reactant Type |
acceptor | |||||||
Mol |
1.34 mmol | |||||||
|
|
|
|||||||
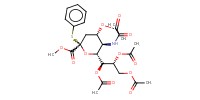
Reactant Type |
donor | |||||||
Mol |
2.01 mmol | |||||||
|
|
|
|||||||
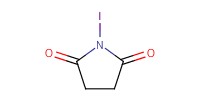
Reactant Type |
NIS | |||||||
Mol |
3.09 mmol | |||||||
|
|

|
|||||||
Reactant Type |
TfOH | |||||||
Mol |
0.54 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
|
|
|||||
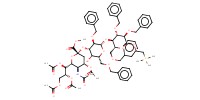
Product Type |
alpha | |||||||
Yield |
60% | |||||||
MOLECULE ID |
|
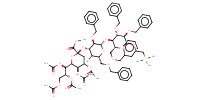
|
|
|||||
Product Type |
beta | |||||||
Yield |
11% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
6 hours | |||||||
Reaction Temp |
-35 degree C | |||||||
Solvent |
dry MeCN | |||||||
Comment |
19, 12, and MS 3A were mixed and stirred for 5 hours at room temperature before the reaction. | |||||||
| MS 3A was included in the solvent. | ||||||||
| COMMENT | ||||||||
| Keywords: synthesis, sialyl glycosides, sialylation, penta-O-benzyl-lactose | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000196 | |||||||
Source |
PREPARATIVE CARBOHYDRATE CHEMISTRY | |||||||
Reference Id |
REF-0000-000197 | |||||||
Source |
ISBN 0-8247-9802-3 | |||||||
Reference Id |
REF-0000-000244 | |||||||
Source |
J. Carbohydr. Chem. 10:493 (1991) | |||||||
Reference Id |
REF-0000-000246 | |||||||
Source |
J. Carbohydr. Chem. 11:699 (1992) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|