
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001762 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001762

|
|||||||
Regist Date |
2012/06/21 17:53:18 | |||||||
| REACTANT | ||||||||
|
|
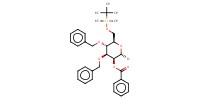
|
|||||||
Reactant Type |
(in 9 mL of CH2Cl2) | |||||||
Mol |
13.5 mmol (or less) | |||||||
|
|
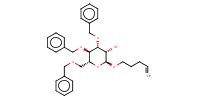
|
|
||||||
Reactant Type |
(in 10 mL of CH2Cl2) | |||||||
Mol |
8.98 mmol | |||||||
|
|

|
|||||||
Reactant Type |
AgOTf (in 15 mL CH2Cl2) | |||||||
Mol |
15.29 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
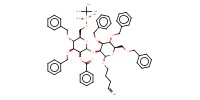
|
|
|||||
Yield |
89% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
10 minutes | |||||||
Reaction Temp |
-40 degree C | |||||||
Solvent |
CH2Cl2 | |||||||
Comment |
MS 4A was included in the solvent. | |||||||
| 82, MS 4A, and AgOTf were mixed and stirred for 5 minutes at -60 degree in Celsius before the reaction. | ||||||||
| COMMENT | ||||||||
| Keywords: oligosaccharide synthesis, n-pentenyl glycosides, 1,2-Orthoesters, Glycosyl Bromides, Coupling, Koenigs-Knorr Procedure | ||||||||
| The reaction is the 2nd part of the sequence. | ||||||||
| ATTENTION: There is a numerical discrepancy between the scheme and the written method. (the reaction time) | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000196 | |||||||
Source |
PREPARATIVE CARBOHYDRATE CHEMISTRY | |||||||
Reference Id |
REF-0000-000197 | |||||||
Source |
ISBN 0-8247-9802-3 | |||||||
Reference Id |
REF-0000-000236 | |||||||
Source |
J. Am. Chem. Soc. 117:1546 (1995) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|