
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001744 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001744

|
||||
Regist Date |
2012/06/21 17:50:04 | ||||
| REACTANT | |||||
|
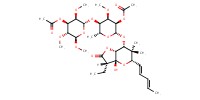
|
|
||||
Mol |
0.039 mmol (at least) | ||||
|
|
|
||||
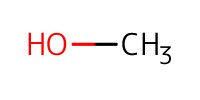
Reactant Type |
MeOH (solvent) | ||||
Volume |
1 mL | ||||
|
|
|
||||
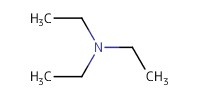
Reactant Type |
Et3N | ||||
Mol |
catalytic amount | ||||
| PRODUCT | |||||
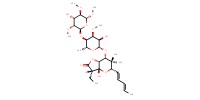
MOLECULE ID |
|
|
|||
Yield |
78%(at least) | ||||
| REACTION DETAIL | |||||
Reaction Time |
48 hours | ||||
Reaction Temp |
room temp | ||||
Solvent |
dry MeOH | ||||
| COMMENT | |||||
| Keywords: glycosyl fluorides, glycosyl sulfides, elfamycin | |||||
| The reaction is the 2nd part of the sequence. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000196 | ||||
Source |
PREPARATIVE CARBOHYDRATE CHEMISTRY | ||||
Reference Id |
REF-0000-000197 | ||||
Source |
ISBN 0-8247-9802-3 | ||||
Reference Id |
REF-0000-000231 | ||||
Source |
J. Am. Chem. Soc. 107:1691 (1985) | ||||
Reference Id |
REF-0000-000232 | ||||
Source |
J. Am. Chem. Soc. 107:1695 (1985) | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|