
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001721 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001721

|
|||||||
Regist Date |
2012/06/21 17:44:06 | |||||||
| REACTANT | ||||||||
|
|
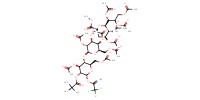
|
|
||||||
Mol |
18 micro mole | |||||||
|
|
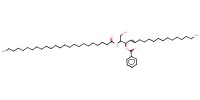
|
|||||||
Mol |
26 mmol | |||||||
|
|
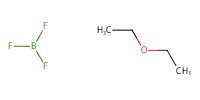
|
|||||||
Reactant Type |
BF3*OEt2 | |||||||
Mol |
23 micro mole | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
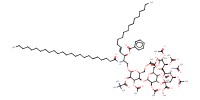
|
|
|||||
Yield |
65% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
3 hours, 12 hours | |||||||
Reaction Temp |
-5 degree C, 20 degree C | |||||||
Solvent |
CHCl3, CHCl3 | |||||||
Comment |
1) +all, 2) temperature change | |||||||
| MS 4A was included in the solvent. | ||||||||
| COMMENT | ||||||||
| Keywords: glycosylation, O-glycosyl trichloroacetimidates | ||||||||
| There are multiple steps in this reaction. | ||||||||
| ATTENTION: CHCl3 is mentioned in the written method, whereas CH2Cl2 is mentioned in the scheme. (solvent) | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000196 | |||||||
Source |
PREPARATIVE CARBOHYDRATE CHEMISTRY | |||||||
Reference Id |
REF-0000-000197 | |||||||
Source |
ISBN 0-8247-9802-3 | |||||||
Reference Id |
REF-0000-000220 | |||||||
Source |
Carbohydr. Res. 203:205 (1990) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|