
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001718 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001718

|
||||
Regist Date |
2012/06/21 17:43:13 | ||||
| REACTANT | |||||
|
|
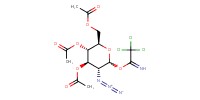
|
||||
Mol |
0.025 mol | ||||
|
|
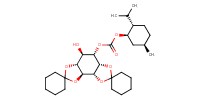
|
||||
Mol |
0.019 mol | ||||
|
|

|
||||
Reactant Type |
TfOH | ||||
Mol |
1.8 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
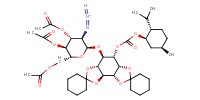
|
|||
Yield |
86% | ||||
| REACTION DETAIL | |||||
Reaction Time |
15 minutes | ||||
Reaction Temp |
room temp | ||||
Solvent |
Et2O/CH2Cl2 = 6/1 | ||||
Comment |
42, 43, and MS 3A were mixed and stirred for 20 minutes at room temperature before the reaction. | ||||
| MS 3A was included in the solvent. | |||||
| COMMENT | |||||
| Keywords: glycosylation, O-glycosyl trichloroacetimidates | |||||
| ATTENTION: There are multiple discrepancies between the book and the reference. (the reagent, the solvent, and the number of % yield) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000196 | ||||
Source |
PREPARATIVE CARBOHYDRATE CHEMISTRY | ||||
Reference Id |
REF-0000-000197 | ||||
Source |
ISBN 0-8247-9802-3 | ||||
Reference Id |
REF-0000-000211 | ||||
Source |
Angew. Chem. 106:2289 (1994) | ||||
Reference Id |
REF-0000-000212 | ||||
Source |
Angew. Chem. Int. Ed. Engl. 33:2177 (1994) | ||||
Reference Id |
REF-0000-000217 | ||||
Source |
Thomas G. Mayer, Dissertation, Universitat Konstanz, 1995, | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|