
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001619 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001619

|
||||
Regist Date |
2012/06/21 17:26:47 | ||||
| REACTANT | |||||
|
|
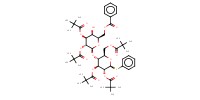
|
||||
|
|
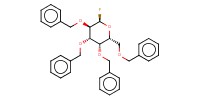
|
||||
|
|

|
||||
Reactant Type |
AgOTf | ||||
|
|
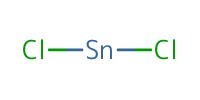
|
||||
Reactant Type |
SnCl2 | ||||
| PRODUCT | |||||
MOLECULE ID |
|
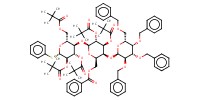
|
|||
Yield |
73% | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified | ||||
Reaction Temp |
0 degree C | ||||
Solvent |
Et2O | ||||
Comment |
Very few were described regarding this reaction. | ||||
| MS 4A was included in the solvent. | |||||
| COMMENT | |||||
| Keywords: synthesis of glycosphingolipid, globotriosylceramide, Gb3 | |||||
| The reaction is the 1st part of the sequence. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000073 | ||||
Source |
第4版 実験化学講座 (26), 丸善 | ||||
Reference Id |
REF-0000-000074 | ||||
Source |
ISBN 4-621-03702-1 | ||||
Reference Id |
REF-0000-000191 | ||||
Source |
K. C. Nicolaou, T. J. Caulfield, and H. Kataoka, Carbohydr. Res., 202, 177 (1990) | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|