
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001612 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001612

|
||||
Regist Date |
2012/06/21 17:26:01 | ||||
| REACTANT | |||||
|
|
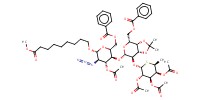
|
||||
|
|
|
||||
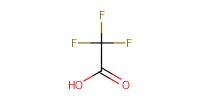
Reactant Type |
TFA (50% in CH2Cl2) | ||||
|
|
|
||||
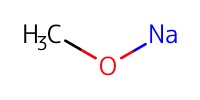
Reactant Type |
NaOMe (in MeOH) | ||||
|
|
|
||||
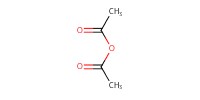
Reactant Type |
Ac2O | ||||
| PRODUCT | |||||
MOLECULE ID |
|
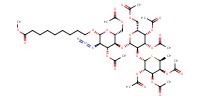
|
|||
Yield |
24%(at least) | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified, NOT specified, NOT specified | ||||
Reaction Temp |
NOT specified, NOT specified, NOT specified | ||||
Solvent |
CH2Cl2, MeOH, pyridine | ||||
Comment |
1) 8+TFA, 2) +NaOMe, 3) +Ac2O | ||||
| Very few were described regarding this reaction. | |||||
| COMMENT | |||||
| Keywords: biological activity, carbohydrates, conformation analysis, lectins, synthesis design | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000189 | ||||
Issn |
|||||
Doi |
10.1002/chem.200400831 | ||||
PubMed ID |
15770712 | ||||
Journal Name |
Chemistry (Weinheim an der Bergstrasse, Germany). (2005) 11 (10): 3032-8. | ||||
Article Title |
Synthesis and evaluation of 5-thio-L-fucose-containing oligosaccharide. | ||||
Author |
Masayuki, Izumi; Osamu, Tsuruta; Yasuhiro, Kajihara; Shin, Yazawa; Hideya, Yuasa; Hironobu, Hashimoto | ||||
Affiliation |
Department of Life Science, Graduate school of Bioscience and Biotechnology, Tokyo Institute of Technology, 4259 Nagatsuta, Yokohama 226-8501, Japan.masayuki.izumi@aist.go.jp | ||||
Reference Id |
REF-0000-000190 | ||||
Source |
Chem. Eur. J. 2005, 11, 3032-3038 | ||||
Doi |
10.1002/chem.200400831 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|