
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001589 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001589

|
|||||||
Regist Date |
2012/06/21 17:23:40 | |||||||
| REACTANT | ||||||||
|
|
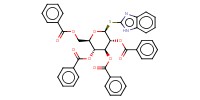
|
|||||||
Reactant Type |
donor | |||||||
Mol |
0.045 mmol | |||||||
|
|
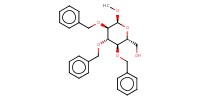
|
|
||||||
Reactant Type |
acceptor | |||||||
Mol |
0.030 mmol | |||||||
|
|
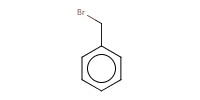
|
|||||||
Reactant Type |
promoter | |||||||
Mol |
0.027 mmol | |||||||
| PRODUCT | ||||||||
| REACTION DETAIL | ||||||||
Reaction Time |
120 hours | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
ClCH2CH2Cl | |||||||
Comment |
The donor, the acceptor, and MS 4A were mixed and stirred for 1 hour before the reaction. | |||||||
| COMMENT | ||||||||
| Keywords: carbohydrates, glycosides, glycosylation, oligosaccharides, synthetic methods | ||||||||
| The reaction did not occur. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000187 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1002/anie.201007212 | |||||||
PubMed ID |
21433229 | |||||||
Journal Name |
Angewandte Chemie (International ed. in English). (2011) 50 (18): 4197-201. | |||||||
Article Title |
S-Benzimidazolyl glycosides as a platform for oligosaccharide synthesis by an active-latent strategy. | |||||||
Author |
Scott J, Hasty; Matthew A, Kleine; Alexei V, Demchenko | |||||||
Affiliation |
Department of Chemistry and Biochemistry, University of Missouri-St.?Louis, One University Boulevard, St.?Louis, MO 63121, USA. | |||||||
Reference Id |
REF-0000-000188 | |||||||
Source |
Angew. Chem. Int. Ed. 2011, 50, 4197-4201 | |||||||
Doi |
10.1002/anie.201007212 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|