
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001532 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001532

|
||||
Regist Date |
2012/06/21 17:17:41 | ||||
| REACTANT | |||||
|
|
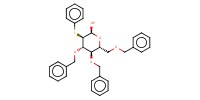
|
||||
|
|

|
||||
Reactant Type |
CCl3CN | ||||
|
|

|
||||
Reactant Type |
NaH | ||||
| PRODUCT | |||||
MOLECULE ID |
|
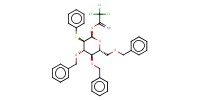
|
|||
Yield |
NOT specified | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified | ||||
Reaction Temp |
NOT specified | ||||
Solvent |
NOT specified | ||||
Comment |
Very few were described regarding this reaction. | ||||
| COMMENT | |||||
| Keywords: The synthesis of 2-deoxy-glycoside, stereoselectivity control using C-2 substitution group | |||||
| The reaction is the 2nd part of the sequence. | |||||
| ATTENTION: There is an error in the chemical structure. (C-2; OBn should be SPh) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000073 | ||||
Source |
第4版 実験化学講座 (26), 丸善 | ||||
Reference Id |
REF-0000-000074 | ||||
Source |
ISBN 4-621-03702-1 | ||||
Reference Id |
REF-0000-000168 | ||||
Source |
R. Preuss and R. R. Schmidt, Synthesis, 1988, 694 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|