
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001488 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001488

|
|||||||
Regist Date |
2012/06/21 17:11:00 | |||||||
| REACTANT | ||||||||
|
|
|
|||||||
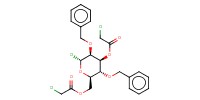
Reactant Type |
chloride | |||||||
|
|
|
|
||||||
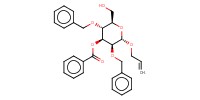
Reactant Type |
alcohol | |||||||
|
|

|
|||||||
Reactant Type |
AgOSO2CF3 | |||||||
|
|
|
|||||||
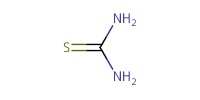
Reactant Type |
(H2N)2CS | |||||||
| PRODUCT | ||||||||
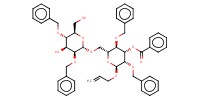
MOLECULE ID |
|
|
|
|||||
Yield |
54% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
16 hours, NOT specified | |||||||
Reaction Temp |
10 to 20 degree C, NOT specified | |||||||
Solvent |
ClCH2CH2Cl, EtOH | |||||||
Comment |
1) chloride+alcohol, MS 4A, AgOSO2CF3, 2) +(H2N)2CS | |||||||
| Very few were described regarding this reaction. | ||||||||
| COMMENT | ||||||||
| Keywords: The synthesis of 1,2-trans-glycoside, alpha-mannopyranoside | ||||||||
| There are multiple steps in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000073 | |||||||
Source |
第4版 実験化学講座 (26), 丸善 | |||||||
Reference Id |
REF-0000-000074 | |||||||
Source |
ISBN 4-621-03702-1 | |||||||
Reference Id |
REF-0000-000135 | |||||||
Source |
T. Ogawa and T. Nukada, Carbohydr. Res., 136, 135 (1985) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|