
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001447 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001447

|
|||||||
Regist Date |
2012/06/21 17:04:19 | |||||||
| REACTANT | ||||||||
|
|
|
|||||||
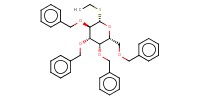
Reactant Type |
thioglycoside | |||||||
Mol |
0.18 mmol | |||||||
|
|
|
|
||||||
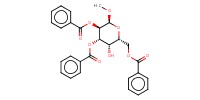
Reactant Type |
alcohol | |||||||
Mol |
0.15 mmol | |||||||
|
|

|
|||||||
Reactant Type |
AgOSO2CF3 | |||||||
Mol |
0.45 mmol | |||||||
|
|
|
|||||||
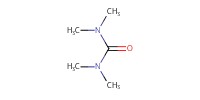
Reactant Type |
(Me2N)2CO | |||||||
Mol |
0.30 mmol | |||||||
|
|
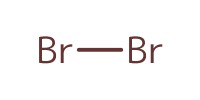
|
|||||||
Reactant Type |
Br2 | |||||||
Mol |
0.11 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
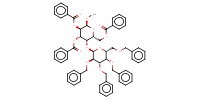
|
|
|||||
Yield |
88% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
20 minutes, 75 minutes | |||||||
Reaction Temp |
room temp, room temp | |||||||
Solvent |
PhCH3, PhCH3 | |||||||
Comment |
1) thioglycoside+MS 4A, alcohol, AgOSO2CF3, (Me2N)2CO, 2) +Br2 | |||||||
| MS 4A was included in the solvent. | ||||||||
| Thioglycoside, alcohol, and MS 4A were mixed and stirred for 4 hours before the reaction. | ||||||||
| COMMENT | ||||||||
| Keywords: The synthesis of 1,2-cis-glycoside, alpha-glucopyranoside | ||||||||
| There are multiple steps in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000073 | |||||||
Source |
第4版 実験化学講座 (26), 丸善 | |||||||
Reference Id |
REF-0000-000074 | |||||||
Source |
ISBN 4-621-03702-1 | |||||||
Reference Id |
REF-0000-000102 | |||||||
Source |
J. O. Kihlberg, D. A. Leigh, and D. R. Bundle, J. Org. Chem., 55, 2860 (1990) | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|