
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001372 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001372

|
||||
Regist Date |
2012/06/21 16:54:53 | ||||
| REACTANT | |||||
|
|
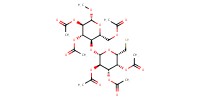
|
||||
Mol |
0.400 mmol | ||||
|
|

|
||||
Reactant Type |
HCl (gas) | ||||
|
|
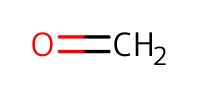
|
||||
Reactant Type |
paraformaldehyde | ||||
Mol |
4.98 equiv. | ||||
|
|
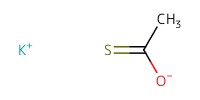
|
||||
Reactant Type |
potassium thioacetate | ||||
Mol |
4.93 equiv. | ||||
| PRODUCT | |||||
MOLECULE ID |
|
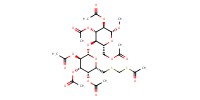
|
|||
Yield |
92% | ||||
| REACTION DETAIL | |||||
Reaction Time |
20 minutes, 3 hours, 2 hours | ||||
Reaction Temp |
0 degree C, 0 degree C, 50 degree C | ||||
Solvent |
CH2Cl2, CH2Cl2, DMF | ||||
Comment |
1) 12b+HCl, paraformaldehyde, 2) allowed to stand, 3) +potassium thioacetate | ||||
| COMMENT | |||||
| Keywords: Bisubstrate-type sialyltransferase inhibitors, CMP-NeuAc, N-acetyllactosamine, lactose, sialic acid, ST6N, ST3N | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000071 | ||||
Issn |
|||||
Doi |
10.1021/jo030042g | ||||
PubMed ID |
12839452 | ||||
Journal Name |
The Journal of organic chemistry. (2003) 68 (14): 5602-13. | ||||
Article Title |
Systematic syntheses and inhibitory activities of bisubstrate-type inhibitors of sialyltransferases. | ||||
Author |
Hiroshi, Hinou; Xue-Long, Sun; Yukishige, Ito | ||||
Affiliation |
RIKEN (The Institute of Physical and Chemical Research), 2-1 Hirosawa, Wako-shi, Saitama 351-0198, Japan. | ||||
Reference Id |
REF-0000-000072 | ||||
Source |
J. Org. Chem. 2003, 68, 5602-5613 | ||||
Doi |
10.1021/jo030042g | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|