
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001363 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001363

|
|||||||
Regist Date |
2012/06/21 16:53:56 | |||||||
| REACTANT | ||||||||
|
|
|
|||||||
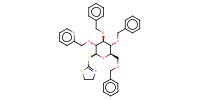
Reactant Type |
donor | |||||||
Mol |
0.13 mmol | |||||||
|
|
|
|
||||||
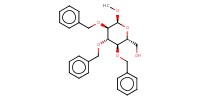
Reactant Type |
acceptor | |||||||
Mol |
0.10 mmol | |||||||
|
|

|
|||||||
Reactant Type |
Cu(OTf)2 | |||||||
Mol |
0.39 mmol | |||||||
|
|
|
|||||||
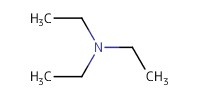
Reactant Type |
Et3N | |||||||
Volume |
0.5 mL | |||||||
| PRODUCT | ||||||||
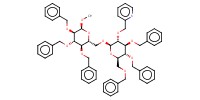
MOLECULE ID |
|
|
|
|||||
Yield |
80% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
16 hours, 30 minutes | |||||||
Reaction Temp |
40 degree C, NOT specified | |||||||
Solvent |
ClCH2CH2Cl, ClCH2CH2Cl | |||||||
Comment |
1) donor+acceptor, MS 4A, Cu(OTf)2, 2) +Et3N | |||||||
| The donor, the acceptor, and MS 4A were mixed and stirred for 1 hour before the reaction. | ||||||||
| COMMENT | ||||||||
| Keywords: carbohydrates, glycosides, glycosylation, oligosaccharides, thioimidates | ||||||||
| There are multiple phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000069 | |||||||
Issn |
||||||||
Doi |
10.1002/anie.200502694 | |||||||
PubMed ID |
16224750 | |||||||
Journal Name |
Angewandte Chemie (International ed. in English). (2005) 44 (43): 7123-6. | |||||||
Article Title |
Development of an arming participating group for stereoselective glycosylation and chemoselective oligosaccharide synthesis. | |||||||
Author |
James T, Smoot; Papapida, Pornsuriyasak; Alexei V, Demchenko | |||||||
Affiliation |
Department of Chemistry and Biochemistry, University of Missouri-St. Louis, One University Boulevard, St. Louis, MO 63121, USA. | |||||||
Reference Id |
REF-0000-000070 | |||||||
Source |
Angew. Chem. Int. Ed. 2005, 44, 7123-7126 | |||||||
Doi |
10.1002/anie.200502694 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|