
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001314 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001314

|
||||
Regist Date |
2012/06/21 16:50:15 | ||||
| REACTANT | |||||
|
|
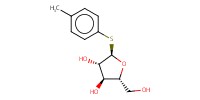
|
||||
Mol |
170 micro mol | ||||
|
|
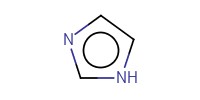
|
||||
Reactant Type |
imidazole | ||||
Mol |
425 micro mol | ||||
|
|
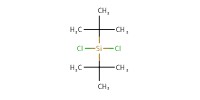
|
||||
Reactant Type |
DTBSCl | ||||
Mol |
178 micro mol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
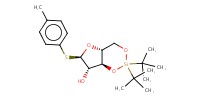
|
|||
Yield |
45% | ||||
| REACTION DETAIL | |||||
Reaction Time |
overnight | ||||
Reaction Temp |
room temp | ||||
Solvent |
dry DMF | ||||
Comment |
The reactants were mixed at 0 degree in Celsius before stirred at room temperature. | ||||
| COMMENT | |||||
| Keywords: stereoselective synthesis, mycobacterial arabinan, arabinofuranosyl donors, arabinofuranosylation, TIDPS | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000067 | ||||
Issn |
|||||
Doi |
10.1021/ol062198j | ||||
PubMed ID |
17107063 | ||||
Journal Name |
Organic letters. (2006) 8 (24): 5525-8. | ||||
Article Title |
Stereoselective synthesis of a fragment of mycobacterial arabinan. | ||||
Author |
Akihiro, Ishiwata; Hiroko, Akao; Yukishige, Ito | ||||
Affiliation |
RIKEN (Institute of Physical and Chemical Research), Wako-shi, Saitama 351-0198, Japan. | ||||
Reference Id |
REF-0000-000068 | ||||
Source |
ORGANIC LETTERS, 2006, Vol. 8, No. 24, 5525-5528 | ||||
Doi |
10.1021/ol062198j | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|