
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001294 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001294

|
||||
Regist Date |
2012/06/21 16:49:18 | ||||
| REACTANT | |||||
|
|
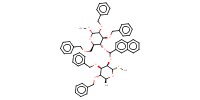
|
||||
Reactant Type |
acetal | ||||
Mol |
1 equiv. | ||||
|
|
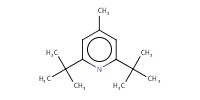
|
||||
Reactant Type |
DTBMP | ||||
Mol |
4 equiv. | ||||
|
|
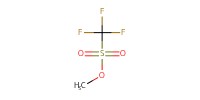
|
||||
Reactant Type |
MeOTf | ||||
Mol |
3.5 equiv. | ||||
|
|
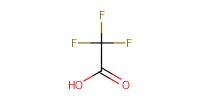
|
||||
Reactant Type |
TFA | ||||
Volume |
200 micro L | ||||
|
|
|
||||
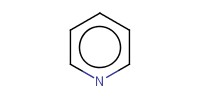
Reactant Type |
pyridine | ||||
Volume |
5.0 mL | ||||
|
|
|
||||
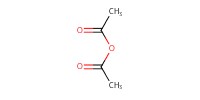
Reactant Type |
Ac2O | ||||
Volume |
500 micro L | ||||
|
|
|
||||
Reactant Type |
DMAP | ||||
Mol |
catalytic amount | ||||
| PRODUCT | |||||
MOLECULE ID |
|
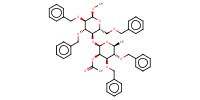
|
|||
Product Type |
beta | ||||
Yield |
72%(beta only) | ||||
| REACTION DETAIL | |||||
Reaction Time |
NOT specified, 3 hours, 12 hours | ||||
Reaction Temp |
50 degree C, 0 degree C, room temp | ||||
Solvent |
(CH2Cl)2, CH2Cl2, CH2Cl2 | ||||
Comment |
1) acetal+DTBMP, MS 4A, MeOTf, 2) +TFA, 3) +all the rest | ||||
| MS 4A was included in the solvent. | |||||
| The reactants were mixed at room temperature before stirred at 50 degree in Celsius. (first phase) | |||||
| COMMENT | |||||
| Keywords: beta-L-rhamnopyranosides, stereoselective synthesis, beta-D-mannopyranosides, intramolecular aglycon delivery, IAD | |||||
| There are multiple phases in this reaction. | |||||
| The alpha/beta ratio was determined by 1H NMR after isolation. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000065 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/ja801574q | ||||
PubMed ID |
18433121 | ||||
Journal Name |
Journal of the American Chemical Society. (2008) 130 (20): 6330-1. | ||||
Article Title |
Stereoselective synthesis of beta-L-rhamnopyranosides. | ||||
Author |
Yong Joo, Lee; Akihiro, Ishiwata; Yukishige, Ito | ||||
Affiliation |
RIKEN (The Institute of Physical and Chemical Research), 2-1 Hirosawa, Wako, Saitama 351-0198, Japan. | ||||
Reference Id |
REF-0000-000066 | ||||
Source |
J. AM. CHEM. SOC. 2008, 130, 6330-6331 | ||||
Doi |
10.1021/ja801574q | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|