
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001256 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001256

|
||||
Regist Date |
2012/06/21 16:47:26 | ||||
| REACTANT | |||||
|
|
|
||||
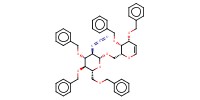
Reactant Type |
glycal (in Ac2O) | ||||
Mol |
2.06 mmol | ||||
|
|
|
||||
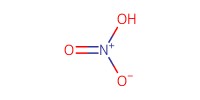
Reactant Type |
HNO3 (in Ac2O) | ||||
Mol |
3.82 equiv. | ||||
|
|
|
||||
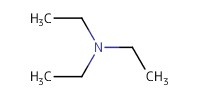
Reactant Type |
TEA (in CH2Cl2) | ||||
Mol |
1.21 equiv. | ||||
| PRODUCT | |||||
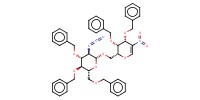
MOLECULE ID |
|
|
|||
Yield |
78% | ||||
| REACTION DETAIL | |||||
Reaction Time |
10 to 15 minutes + 1 hour, 25 minutes | ||||
Reaction Temp |
-30 degree C, room temp | ||||
Solvent |
Ac2O, CH2Cl2 | ||||
Comment |
1) glycal+HNO3, 2) +TEA | ||||
| Before the reaction, concentrated HNO3 was added dropwise to Ac2O at 10 degree in Celsius under constant stirring. | |||||
| The glycal was added over a period of 10 to 15 minutes. | |||||
| The reactants were mixed at 0 degree in Celsius, and allowed to warm slowly to room temperature within the reaction time. (second phase) | |||||
| COMMENT | |||||
| Keywords: galactal derivatives, 6-O-TIPS protection, trichloroacetimidates, Michael-type addition, 2-nitroglycals | |||||
| There are multiple phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000063 | ||||
Issn |
|||||
Doi |
10.1021/jo061670b | ||||
PubMed ID |
17503844 | ||||
Journal Name |
The Journal of organic chemistry. (2007) 72 (12): 4367-77. | ||||
Article Title |
Glycal glycosylation and 2-nitroglycal concatenation, a powerful combination for mucin core structure synthesis. | ||||
Author |
Jürgen, Geiger; B Gopal, Reddy; Gottfried A, Winterfeld; R, Weber; M, Przybylski; R R, Schmidt | ||||
Affiliation |
Fachbereich Chemie, Universität Konstanz, Fach M 725, D - 78457 Konstanz, Germany. | ||||
Reference Id |
REF-0000-000064 | ||||
Source |
J. Org. Chem. 2007, 72, 4367-4377 | ||||
Doi |
10.1021/jo061670b | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|