
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001251 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001251

|
||||
Regist Date |
2012/06/21 16:47:13 | ||||
| REACTANT | |||||
|
|
|
||||
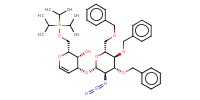
Mol |
0.046 mmol | ||||
|
|

|
||||
Reactant Type |
NaH | ||||
Mol |
0.1 mmol | ||||
|
|
|
||||
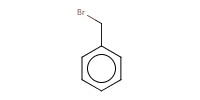
Reactant Type |
BnBr | ||||
Mol |
1.0 mmol | ||||
| PRODUCT | |||||
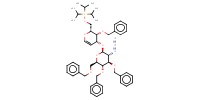
MOLECULE ID |
|
|
|||
Yield |
55% | ||||
| REACTION DETAIL | |||||
Reaction Time |
15 minutes, NOT specified | ||||
Reaction Temp |
NOT specified, NOT specified | ||||
Solvent |
DMF, DMF | ||||
Comment |
1) 25A+NaH, 2) +BnBr | ||||
| BnBr was added dropwise. | |||||
| COMMENT | |||||
| Keywords: galactal derivatives, 6-O-TIPS protection, trichloroacetimidates, Michael-type addition, 2-nitroglycals | |||||
| There are multiple phases in this reaction. | |||||
| ATTENTION: There are numerical discrepancies between the scheme and the written method. (the number of % yield) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000063 | ||||
Issn |
|||||
Doi |
10.1021/jo061670b | ||||
PubMed ID |
17503844 | ||||
Journal Name |
The Journal of organic chemistry. (2007) 72 (12): 4367-77. | ||||
Article Title |
Glycal glycosylation and 2-nitroglycal concatenation, a powerful combination for mucin core structure synthesis. | ||||
Author |
Jürgen, Geiger; B Gopal, Reddy; Gottfried A, Winterfeld; R, Weber; M, Przybylski; R R, Schmidt | ||||
Affiliation |
Fachbereich Chemie, Universität Konstanz, Fach M 725, D - 78457 Konstanz, Germany. | ||||
Reference Id |
REF-0000-000064 | ||||
Source |
J. Org. Chem. 2007, 72, 4367-4377 | ||||
Doi |
10.1021/jo061670b | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|