
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001171 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-001171

|
|||||||
Regist Date |
2012/06/21 16:43:00 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
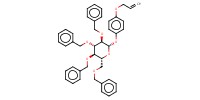
Mol |
0.45 mmol | |||||||
|
|
|
|||||||
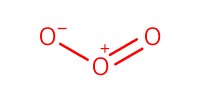
Reactant Type |
O3 (gas) | |||||||
|
|
|
|||||||
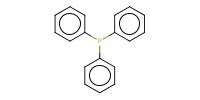
Reactant Type |
Ph3P | |||||||
Mol |
1.44 mmol | |||||||
| PRODUCT | ||||||||
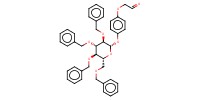
MOLECULE ID |
|
|
|
|||||
Yield |
97% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
10 minutes, 3 hours | |||||||
Reaction Temp |
-78 degree C, room temp | |||||||
Solvent |
CH2Cl2, CH2Cl2 | |||||||
Comment |
1) 6+O3, 2) +Ph3P | |||||||
| The reactants were mixed at -78 degree in Celsius, and allowed to warm to room temperature. (second phase) | ||||||||
| COMMENT | ||||||||
| Keywords: cyclodextrin, drug delivery system, drug carrier, stacking effect, arbutin, doxorubicin | ||||||||
| The DOI could not be found. | ||||||||
| There are two phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000059 | |||||||
Issn |
||||||||
PubMed ID |
18473917 | |||||||
Journal Name |
Medicinal chemistry (Shāriqah (United Arab Emirates)). (2008) 4 (3): 244-55. | |||||||
Article Title |
Beta-cyclodextrin conjugates with glucose moieties designed as drug carriers: their syntheses, evaluations using concanavalin A and doxorubicin, and structural analyses by NMR spectroscopy. | |||||||
Author |
Yoshiki, Oda; Natsumi, Kobayashi; Takashi, Yamanoi; Kaname, Katsuraya; Keiko, Takahashi; Kenjiro, Hattori | |||||||
Affiliation |
The Noguchi Institute, 1-8-1 Kaga, Itabashi-ku, Tokyo 173-0003, Japan. | |||||||
Reference Id |
REF-0000-000060 | |||||||
Source |
Medicinal Chemistry, 2008, 4, 244-255 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|