
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001156 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001165

|
||||
Regist Date |
2012/06/21 16:42:45 | ||||
| REACTANT | |||||
|
|
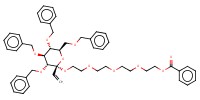
|
||||
Mol |
0.26 mmol | ||||
|
|
|
||||
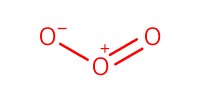
Reactant Type |
O3 (gas) | ||||
|
|
|
||||
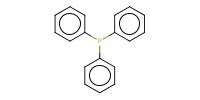
Reactant Type |
Ph3P | ||||
Mol |
0.88 mmol | ||||
|
|
|
||||
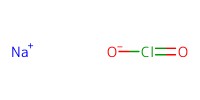
Reactant Type |
NaClO2 | ||||
Mol |
3.1 mmol | ||||
|
|
|
||||
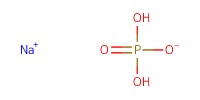
Reactant Type |
NaH2PO4 | ||||
Mol |
0.8 mmol | ||||
|
|
|
||||
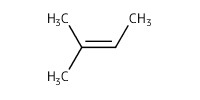
Reactant Type |
2-methyl-2-butene | ||||
Mol |
1.2 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
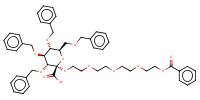
|
|||
Yield |
85% | ||||
| REACTION DETAIL | |||||
Reaction Time |
5 hours, 19 hours, 24 hours | ||||
Reaction Temp |
-78 degree C, room temp, NOT specified | ||||
Solvent |
CH2Cl2, CH2Cl2, t-BuOH/H2O = 4mL/1mL | ||||
Comment |
1) 4+O3, 2) +Ph3P, 3) +all the rest | ||||
| The reactants were mixed at -78 degree in Celsius, and allowed to warm to room temperature. (second phase) | |||||
| COMMENT | |||||
| Keywords: Crown ether, Spiroketal, 1-C-Vinylated glucose, Glycosylation | |||||
| There are three phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000056 | ||||
Issn |
Electronic | ||||
PubMed ID |
18794788 | ||||
Journal Name |
Molecules (Basel, Switzerland). (2008) 13 (8): 1840-5. | ||||
Article Title |
Synthesis of a novel D-glucose-conjugated 15-crown-5 ether with a spiro ketal structure. | ||||
Author |
Takashi, Yamanoi; Yoshiki, Oda; Hitomi, Muraishi; Sho, Matsuda | ||||
Affiliation |
The Noguchi Institute, 1-8-1 Kaga, Itabashi-ku, Tokyo 173-0003, Japan. tyama@noguchi.or.jp | ||||
Reference Id |
REF-0000-000057 | ||||
Source |
Molecules 2008, 13, 1840-1845 manuscripts | ||||
Doi |
10.3390/molecules13081840 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|