
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001134 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001134

|
||||
Regist Date |
2012/06/21 16:40:49 | ||||
| REACTANT | |||||
|
|
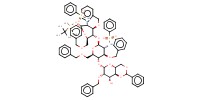
|
||||
|
|
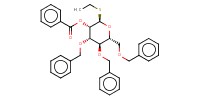
|
||||
|
|
|
||||
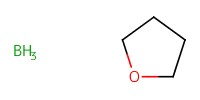
Reactant Type |
BH3*THF | ||||
|
|
|
||||
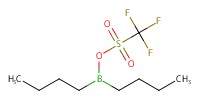
Reactant Type |
Bu2BOTf | ||||
|
|
|
||||
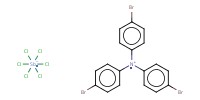
Reactant Type |
(BrC6H4)3NSbCl6 | ||||
|
|
|
||||
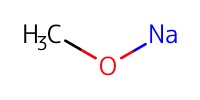
Reactant Type |
NaOMe | ||||
| PRODUCT | |||||
MOLECULE ID |
|
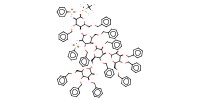
|
|||
Yield |
47.4%(72%, 74%, 89%) | ||||
| REACTION DETAIL | |||||
Solvent |
THF, MeCN, MeOH | ||||
Comment |
1) 6+BH3*THF, Bu2BOTf, 2) +(BrC6H4)3NSbCl6, 3) +NaOMe | ||||
| Very few were described regarding this reaction. | |||||
| COMMENT | |||||
| Keywords: cancer, prostate gland, prostatic epithelium, prostate specific antigen, PSA, amino acid residues | |||||
| There are three phases in this reaction. | |||||
| (BrC6H4)3NSbCl6 = Tris(4-bromophenyl)aminium Hexachloroantimonate (CAS number: 24964-91-8) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000054 | ||||
Issn |
|||||
Doi |
10.1021/ja037988s | ||||
PubMed ID |
14733546 | ||||
Journal Name |
Journal of the American Chemical Society. (2004) 126 (3): 736-8. | ||||
Article Title |
Chemical synthesis of normal and transformed PSA glycopeptides. | ||||
Author |
Vadim Y, Dudkin; Justin S, Miller; Samuel J, Danishefsky | ||||
Affiliation |
Laboratory for Bioorganic Chemistry, The Sloan-Kettering Institute for Cancer Research, 1275 York Avenue, New York, New York 10021, USA. | ||||
Reference Id |
REF-0000-000055 | ||||
Source |
J. Am. Chem. Soc., 2004, 126 (3), pp 736-738 | ||||
Doi |
10.1021/ja037988s | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|