
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001071 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001078

|
||||
Regist Date |
2012/06/21 16:38:02 | ||||
| REACTANT | |||||
|
|
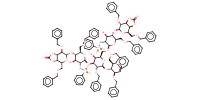
|
||||
Mol |
1 equiv. | ||||
|
|
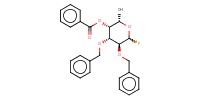
|
||||
Mol |
2.5 equiv. | ||||
|
|
|
||||
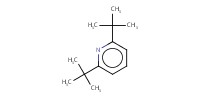
Reactant Type |
di-tert-butylpyridine | ||||
|
|

|
||||
Reactant Type |
Sn(OTf)2 | ||||
| PRODUCT | |||||
MOLECULE ID |
|
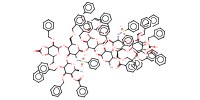
|
|||
Yield |
30% | ||||
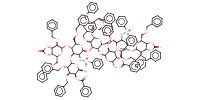
MOLECULE ID |
|
|
|||
Product Type |
difucosyl octasaccharide | ||||
Yield |
55%(total) | ||||
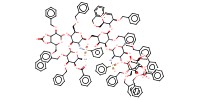
MOLECULE ID |
|
|
|||
Product Type |
difucosyl octasaccharide | ||||
Yield |
55%(total) | ||||
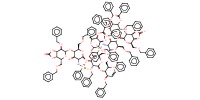
MOLECULE ID |
|
|
|||
Product Type |
difucosyl octasaccharide | ||||
Yield |
55%(total) | ||||
| REACTION DETAIL | |||||
Solvent |
THF/PhCH3 | ||||
Comment |
All the possible structures for compound 53 were listed. | ||||
| Very few were described regarding this reaction. | |||||
| COMMENT | |||||
| ATTENTION: There are numerical discrepancies between the table and the written method. (the number of equiv.; 2.5 or 4) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000048 | ||||
Issn |
|||||
PubMed ID |
11273599 | ||||
Journal Name |
Journal of the American Chemical Society. (2001) 123 (1): 35-48. | ||||
Article Title |
Total syntheses of tumor-related antigens N3: probing the feasibility limits of the glycal assembly method. | ||||
Author |
H M, Kim; I J, Kim; S J, Danishefsky | ||||
Affiliation |
Laboratory for Bioorganic Chemistry, Sloan-Kettering Institute for Cancer Research, 1275 York Ave., New York, New York 10021, USA. | ||||
Reference Id |
REF-0000-000049 | ||||
Source |
J. Am. Chem. Soc. 2001, 123, 35-48 | ||||
Doi |
10.1021/ja0022730 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|