
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0001069 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-001076

|
||||
Regist Date |
2012/06/21 16:37:55 | ||||
| REACTANT | |||||
|
|
|
||||
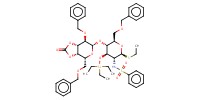
Reactant Type |
azeotropically dried thioglycoside | ||||
Mol |
0.241 mmol | ||||
|
|
|
||||
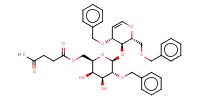
Reactant Type |
lactal | ||||
Mol |
0.135 mmol | ||||
|
|
|
||||
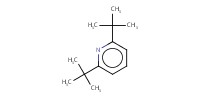
Reactant Type |
di-tert-butylpyridine | ||||
Mol |
0.484 mmol | ||||
|
|
|
||||
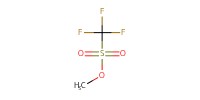
Reactant Type |
MeOTf | ||||
Mol |
0.963 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
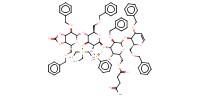
|
|||
Yield |
70% | ||||
| REACTION DETAIL | |||||
Reaction Time |
10 minutes, 6 hours | ||||
Reaction Temp |
room temp, -40 degree C to room temp | ||||
Solvent |
CH2Cl2/Et2O = 3mL/6mL, CH2Cl2/Et2O = 3mL/6mL | ||||
Comment |
1) 47+43, MS 4A, di-tert-butylpyridine, 2) +MeOTf | ||||
| MS 4A was included in the solvent. | |||||
| The reactants were mixed at -40 degree in Celsius, and allowed to warm to room temperature slowly within the reaction time. (second phase) | |||||
| COMMENT | |||||
| ATTENTION: There are numerical discrepancies between the scheme and the written method. (the number of % yield) | |||||
| There might be a typo in Supporting Info. (1,4-di-tert-butylpyridine should be 2,6-di-tert-butylpyridine) | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000048 | ||||
Issn |
|||||
PubMed ID |
11273599 | ||||
Journal Name |
Journal of the American Chemical Society. (2001) 123 (1): 35-48. | ||||
Article Title |
Total syntheses of tumor-related antigens N3: probing the feasibility limits of the glycal assembly method. | ||||
Author |
H M, Kim; I J, Kim; S J, Danishefsky | ||||
Affiliation |
Laboratory for Bioorganic Chemistry, Sloan-Kettering Institute for Cancer Research, 1275 York Ave., New York, New York 10021, USA. | ||||
Reference Id |
REF-0000-000049 | ||||
Source |
J. Am. Chem. Soc. 2001, 123, 35-48 | ||||
Doi |
10.1021/ja0022730 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|