
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000996 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-000997

|
|||||||
Regist Date |
2012/06/21 16:33:43 | |||||||
| REACTANT | ||||||||
|
|
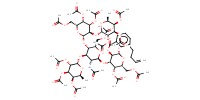
|
|
||||||
Mol |
0.521 mmol | |||||||
|
|
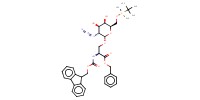
|
|||||||
Mol |
0.104 mmol | |||||||
|
|
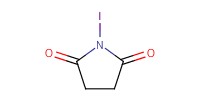
|
|||||||
Reactant Type |
NIS | |||||||
Mol |
1.30 mmol | |||||||
|
|

|
|||||||
Reactant Type |
TfOH (1% in CH2Cl2) | |||||||
Mol |
0.521 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
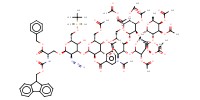
|
||||||
Yield |
69% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
30 minutes, 12 hours | |||||||
Reaction Temp |
room temp, room temp | |||||||
Solvent |
CH2Cl2, CH2Cl2 | |||||||
Comment |
1) 23+19, MS 4A, 2) +all the rest | |||||||
| MS 4A was included in the solvent. | ||||||||
| The reactants were mixed at 0 degree in Celsius, and allowed to raise to room temperature within the reaction time. (second phase) | ||||||||
| COMMENT | ||||||||
| The PMID could not be found. | ||||||||
| There are two phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000046 | |||||||
Source |
J. Am. Chem. Soc. 2000, 122, 7273-7279 | |||||||
Doi |
10.1021/ja0011820 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|