
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000854 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000854

|
||||
Regist Date |
2012/06/21 16:26:40 | ||||
| REACTANT | |||||
|
|
|
||||
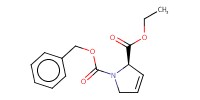
Reactant Type |
dehydro | ||||
Mol |
1.48 mmol | ||||
|
|

|
||||
Reactant Type |
H2O (solvent) | ||||
Volume |
3 mL | ||||
|
|
|
||||
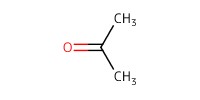
Reactant Type |
acetone (solvent) | ||||
Volume |
1.2 mL | ||||
|
|
|
||||
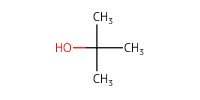
Reactant Type |
t-BuOH (solvent) | ||||
Volume |
0.48 mL | ||||
|
|
|
||||
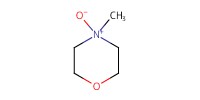
Reactant Type |
NMO | ||||
Mol |
4.43 mmol | ||||
|
|
|
||||
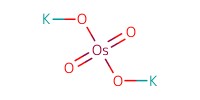
Reactant Type |
K2OsO4 | ||||
Mol |
catalytic amount | ||||
|
|
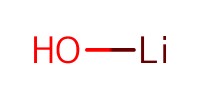
|
||||
Reactant Type |
LiOH (0.1 N) | ||||
Mol |
2 equiv. | ||||
| PRODUCT | |||||
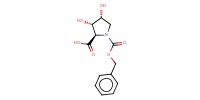
MOLECULE ID |
|
|
|||
Product Type |
N-Cbz-3,4-dihydroxy-D-proline | ||||
Yield |
60% | ||||
| REACTION DETAIL | |||||
Reaction Time |
overnight, 4 hours | ||||
Reaction Temp |
0 degree C, room temp | ||||
Solvent |
H2O/acetone/t-BuOH = 6.25/2.5/1, THF/H2O = 1/1 | ||||
Comment |
1) +all reactants except LiOH, 2) +LiOH | ||||
| COMMENT | |||||
| The PMID could not be found. | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000038 | ||||
Source |
J. Am. Chem. Soc. 1996, 118, 6826-6840 | ||||
Doi |
10.1021/ja952265x | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|