
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000851 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000851

|
||||
Regist Date |
2012/06/21 16:26:29 | ||||
| REACTANT | |||||
|
|
|
||||
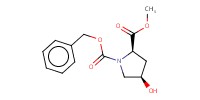
Reactant Type |
N-Cbz-4-hydroxy-D-proline methyl ester | ||||
Mol |
4.4 mmol | ||||
|
|
|
||||
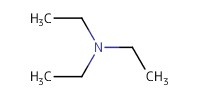
Reactant Type |
Et3N | ||||
Mol |
4.87 mmol | ||||
|
|
|
||||
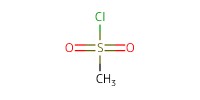
Reactant Type |
MsCl | ||||
Mol |
5.76 mmol | ||||
|
|
|
||||
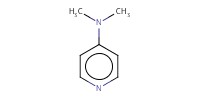
Reactant Type |
DMAP | ||||
Mol |
catalytic amount | ||||
|
|
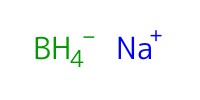
|
||||
Reactant Type |
NaBH4 | ||||
Mol |
0.7 mmol | ||||
|
|
|
||||
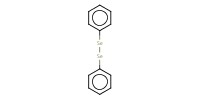
Reactant Type |
PhSe-SePh | ||||
Mol |
0.35 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
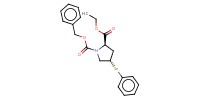
|
|||
Product Type |
(phenylselenyl)proline derivative | ||||
Yield |
63% | ||||
| REACTION DETAIL | |||||
Reaction Time |
1.5 hours, 2 hours | ||||
Reaction Temp |
room temp, reflux | ||||
Solvent |
CH2Cl2, EtOH | ||||
Catalyst |
DMAP | ||||
Comment |
1) N-Cbz-4-hydroxy-D-proline methyl ester+Et3N, MsCl, DMAP, 2) +NaBH4, PhSe-SePh | ||||
| The reactants were mixed at 0 degree in Celsius, and gradually raised to room temperature within the reaction time. (first phase) | |||||
| NaBH4 and PhSe-SePh were mixed independently and stirred for 5 minutes before poured into the solution from the first phase. | |||||
| COMMENT | |||||
| The PMID could not be found. | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000038 | ||||
Source |
J. Am. Chem. Soc. 1996, 118, 6826-6840 | ||||
Doi |
10.1021/ja952265x | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|