
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000735 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000735

|
||||
Regist Date |
2012/06/21 16:21:01 | ||||
| REACTANT | |||||
|
|

|
||||
Mol |
3.33 mmol | ||||
|
|

|
||||
Reactant Type |
Zn (powder) | ||||
Weight |
25.2g | ||||
|
|
|
||||
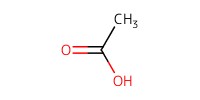
Reactant Type |
AcOH | ||||
Volume |
60 mL | ||||
|
|
|
||||
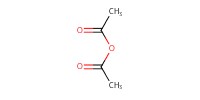
Reactant Type |
Ac2O | ||||
Mol |
5.00 mmol | ||||
| PRODUCT | |||||
MOLECULE ID |
|
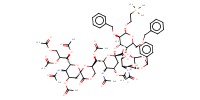
|
|||
Yield |
96% | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour, 30 minutes | ||||
Reaction Temp |
40 degree C, room temp | ||||
Solvent |
CH2Cl2, CH2Cl2 | ||||
Catalyst |
promoter, promoter | ||||
Comment |
1) 10+Zn, AcOH, 2) +Ac2O | ||||
| The reactants were mixed at room temperature before stirred at 40 degree in Celsius. (first phase) | |||||
| COMMENT | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000035 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo8027888 | ||||
PubMed ID |
19296672 | ||||
Journal Name |
The Journal of organic chemistry. (2009) 74 (8): 3009-23. | ||||
Article Title |
Ganglioside GQ1b: efficient total synthesis and the expansion to synthetic derivatives to elucidate its biological roles. | ||||
Author |
Akihiro, Imamura; Hiromune, Ando; Hideharu, Ishida; Makoto, Kiso | ||||
Affiliation |
Department of Applied Bioorganic Chemistry, Faculty of Applied Biological Sciences, Gifu University, 1-1 Yanagido, Gifu-shi, Gifu 501-1193, Japan. aimamura@ualberta.ca | ||||
Reference Id |
REF-0000-000036 | ||||
Source |
J. Org. Chem. 2009, 74, 3009-3023 | ||||
Doi |
10.1021/jo8027888 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|