
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000728 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000728

|
||||
Regist Date |
2012/06/21 16:20:40 | ||||
| REACTANT | |||||
|
|
|
||||
|
|

|
||||
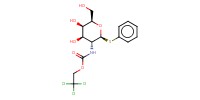
Reactant Type |
PhCH(OMe)2 | ||||
|
|
|
||||
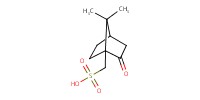
Reactant Type |
CSA | ||||
|
|
|
||||
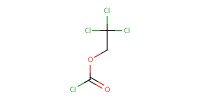
Reactant Type |
TrocCl | ||||
| PRODUCT | |||||
MOLECULE ID |
|

|
|||
Yield |
90% | ||||
| REACTION DETAIL | |||||
Solvent |
MeCN/THF = 2/1, Py | ||||
Comment |
1) 7+PhCH(OMe)2, CSA, 2) +TrocCl | ||||
| Very few were described regarding this reaction. | |||||
| COMMENT | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000035 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jo8027888 | ||||
PubMed ID |
19296672 | ||||
Journal Name |
The Journal of organic chemistry. (2009) 74 (8): 3009-23. | ||||
Article Title |
Ganglioside GQ1b: efficient total synthesis and the expansion to synthetic derivatives to elucidate its biological roles. | ||||
Author |
Akihiro, Imamura; Hiromune, Ando; Hideharu, Ishida; Makoto, Kiso | ||||
Affiliation |
Department of Applied Bioorganic Chemistry, Faculty of Applied Biological Sciences, Gifu University, 1-1 Yanagido, Gifu-shi, Gifu 501-1193, Japan. aimamura@ualberta.ca | ||||
Reference Id |
REF-0000-000036 | ||||
Source |
J. Org. Chem. 2009, 74, 3009-3023 | ||||
Doi |
10.1021/jo8027888 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|