
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000662 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000662

|
||||
Regist Date |
2012/06/21 16:17:29 | ||||
| REACTANT | |||||
|
|
|
||||
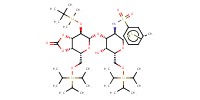
Reactant Type |
thioglycoside | ||||
Mol |
0.12 mmol | ||||
|
|
|
||||
Reactant Type |
alcohol | ||||
Mol |
0.12 mmol | ||||
|
|
|
||||
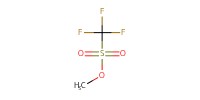
Reactant Type |
MeOTf | ||||
Mol |
0.18 mmol (twice) | ||||
|
|
|
||||
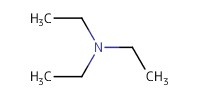
Reactant Type |
Et3N | ||||
Volume |
0.4 mL | ||||
| PRODUCT | |||||
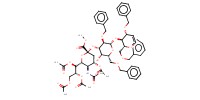
MOLECULE ID |
|
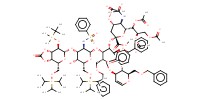
|
|||
Yield |
54% | ||||
MOLECULE ID |
|
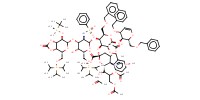
|
|||
Yield |
14% | ||||
| REACTION DETAIL | |||||
Reaction Time |
15 minutes, 36 hours | ||||
Reaction Temp |
room temp, 0 to 5 degree C, | ||||
Solvent |
Et2O/CH2Cl2 = 2mL/1mL, Et2O/CH2Cl2 = 2mL/1mL, | ||||
Comment |
1) 3+8, MS 4A, 2) +MeOTf, Et3N | ||||
| MS 4A was included in the solvent. | |||||
| The second addition of MeOTf was performed 24 hours after the start of the second phase. | |||||
| Et3N was added at the end of the reaction. | |||||
| COMMENT | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000030 | ||||
Issn |
|||||
PubMed ID |
10813908 | ||||
Journal Name |
The Journal of organic chemistry. (2000) 65 (1): 144-51. | ||||
Article Title |
A total synthesis of the methyl glycoside of ganglioside GM(1). | ||||
Author |
S K, Bhattacharya; S J, Danishefsky | ||||
Affiliation |
Department of Chemistry, Columbia University, Havemeyer Hall, New York, New York 10027, USA. | ||||
Reference Id |
REF-0000-000031 | ||||
Source |
J. Org. Chem., 2000, 65, 144-151 | ||||
Doi |
10.1021/jo9912496 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|