
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000659 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000659

|
||||
Regist Date |
2012/06/21 16:17:20 | ||||
| REACTANT | |||||
|
|
|
||||
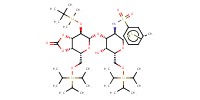
Reactant Type |
thioglycoside | ||||
Mol |
0.19 mmol | ||||
|
|
|
||||
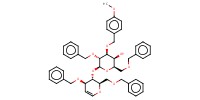
Reactant Type |
alcohol | ||||
Mol |
0.19 mmol | ||||
|
|
|
||||
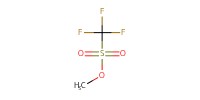
Reactant Type |
MeOTf | ||||
Mol |
0.97 mmol | ||||
|
|
|
||||
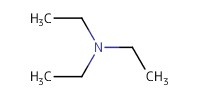
Reactant Type |
Et3N | ||||
Volume |
0.4 mL | ||||
| PRODUCT | |||||
MOLECULE ID |
|
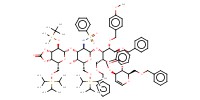
|
|||
Yield |
62%/89% (based on compound 3/4) | ||||
| REACTION DETAIL | |||||
Reaction Time |
15 minutes, 12 hours | ||||
Reaction Temp |
room temp, 0 to 5 degree C | ||||
Solvent |
CH2Cl2/Et2O = 1.5mL/3mL, CH2Cl2/Et2O = 1.5mL/3mL | ||||
Comment |
1) 3+4, MS 4A, 2) +MeOTf, Et3N | ||||
| Compound 3 and 4 were weighed in an oven-dried flask and were then azeotropically dried using anhydrous benzene and thereafter placed under vacuum overnight before the reaction. | |||||
| MS 4A was included in the solvent. (first phase) | |||||
| Et3N was added at the end of the reaction. | |||||
| COMMENT | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000030 | ||||
Issn |
|||||
PubMed ID |
10813908 | ||||
Journal Name |
The Journal of organic chemistry. (2000) 65 (1): 144-51. | ||||
Article Title |
A total synthesis of the methyl glycoside of ganglioside GM(1). | ||||
Author |
S K, Bhattacharya; S J, Danishefsky | ||||
Affiliation |
Department of Chemistry, Columbia University, Havemeyer Hall, New York, New York 10027, USA. | ||||
Reference Id |
REF-0000-000031 | ||||
Source |
J. Org. Chem., 2000, 65, 144-151 | ||||
Doi |
10.1021/jo9912496 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|