
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000613 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000613

|
||||
Regist Date |
2012/06/21 16:15:07 | ||||
| REACTANT | |||||
|
|
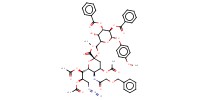
|
||||
Mol |
0.8 mmol | ||||
|
|

|
||||
Reactant Type |
LiOH*H2O (in 5mL H2O) | ||||
Mol |
4.8 mmol | ||||
|
|
|
||||
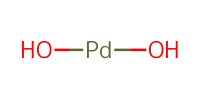
Reactant Type |
Pd(OH)2 (on carbon) | ||||
Weight |
0.9g | ||||
|
|
|
||||
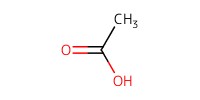
Reactant Type |
AcOH | ||||
Volume |
80 micro L | ||||
|
|
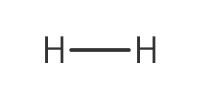
|
||||
Reactant Type |
H2 (gas) | ||||
| PRODUCT | |||||
MOLECULE ID |
|
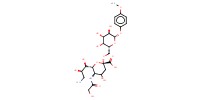
|
|||
Yield |
84.5% | ||||
| REACTION DETAIL | |||||
Reaction Time |
6 hours, 24 hours | ||||
Reaction Temp |
room temp, 50 degree C | ||||
Solvent |
EtOH (20mL), EtOH/H2O = 1/1 (20mL) | ||||
Comment |
1) 6+LiOH*H2O, 2) +Pd(OH)2, H2 | ||||
| The reaction mixture was treated with acidic Dowex 50 at the end of the first phase. | |||||
| COMMENT | |||||
| There are two phases in this reaction. | |||||
| ATTENTION: There are numerical discrepancies between the scheme and the written method. (the second phase temperature, time) | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000028 | ||||
Issn |
Electronic | ||||
Doi |
10.1021/jm8000696 | ||||
PubMed ID |
18841881 | ||||
Journal Name |
Journal of medicinal chemistry. (2008) 51 (21): 6665-81. | ||||
Article Title |
Design, synthesis, and structure-affinity relationships of novel series of sialosides as CD22-specific inhibitors. | ||||
Author |
Hajjaj H M, Abdu-Allah; Taichi, Tamanaka; Jie, Yu; Lu, Zhuoyuan; Magesh, Sadagopan; Takahiro, Adachi; Takeshi, Tsubata; Soerge, Kelm; Hideharu, Ishida; Makoto, Kiso | ||||
Affiliation |
Department of Applied Bio-organic Chemistry, The United Graduate School of Agricultural Sciences, Gifu University, Gifu 501-1193, Japan. | ||||
Reference Id |
REF-0000-000029 | ||||
Source |
J. Med. Chem., 2008, 51, 6665-6681 | ||||
Doi |
10.1021/jm8000696 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|