
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000526 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-000527

|
|||||||
Regist Date |
2012/06/21 16:10:55 | |||||||
| REACTANT | ||||||||
|
|
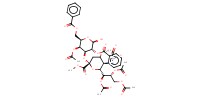
|
|||||||
Mol |
132 micro mole | |||||||
|
|
|
|||||||
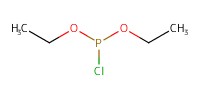
Reactant Type |
ClP(OEt)2 | |||||||
Mol |
266 micro mole | |||||||
|
|
|
|||||||
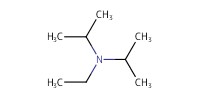
Reactant Type |
DIEA | |||||||
Mol |
398 micro mole | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
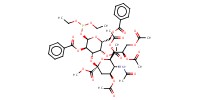
|
|
|||||
Product Type |
alpha | |||||||
Yield |
73%(alpha/beta=1/1.8) | |||||||
MOLECULE ID |
|
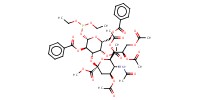
|
|
|||||
Product Type |
beta | |||||||
Yield |
73%(alpha/beta=1/1.8) | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
1 hour | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
CH3CN | |||||||
Comment |
The reactants were mixed at 0 degree in Celsius before stirred at room temperature. | |||||||
| COMMENT | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000021 | |||||||
Issn |
Electronic | |||||||
Doi |
10.1016/j.carres.2009.06.009 | |||||||
PubMed ID |
19560125 | |||||||
Journal Name |
Carbohydrate research. (2009) 344 (12): 1453-63. | |||||||
Article Title |
Study on systematizing the synthesis of the a-series ganglioside glycans GT1a, GD1a, and GM1 using the newly developed N-Troc-protected GM3 and GalN intermediates. | |||||||
Author |
Tatsuya, Komori; Akihiro, Imamura; Hiromune, Ando; Hideharu, Ishida; Makoto, Kiso | |||||||
Affiliation |
Department of Applied Bioorganic Chemistry, Faculty of Applied Biological Sciences, Gifu University, 1-1 Yanagido, Gifu-shi, Gifu 501-1193, Japan. | |||||||
Reference Id |
REF-0000-000022 | |||||||
Source |
Carbohydrate Research, 344 (2009), 1453-1463 | |||||||
Doi |
10.1016/j.carres.2009.06.009 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|