
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000520 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000521

|
||||
Regist Date |
2012/06/21 16:10:35 | ||||
| REACTANT | |||||
|
|
|
||||
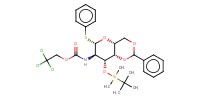
Reactant Type |
Novel galactosaminyl donor | ||||
Mol |
93 micro mole | ||||
|
|
|
||||
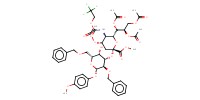
Reactant Type |
sialyl-alpha-(2,3)-galactosyl acceptor | ||||
Mol |
46 micro mole | ||||
|
|
|
||||
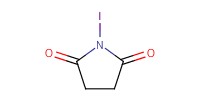
Reactant Type |
NIS | ||||
Mol |
186 micro mole | ||||
|
|

|
||||
Reactant Type |
TfOH | ||||
Mol |
18 micro mole | ||||
| PRODUCT | |||||
MOLECULE ID |
|
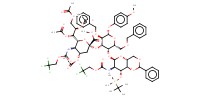
|
|||
Product Type |
alpha | ||||
Yield |
90%(alpha/beta=1/1.0) | ||||
MOLECULE ID |
|
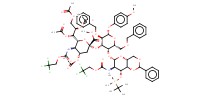
|
|||
Product Type |
beta | ||||
Yield |
90%(alpha/beta=1/1.0) | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour, 1.5 hours | ||||
Reaction Temp |
room temp, room temp | ||||
Solvent |
CH2Cl2 | ||||
Catalyst |
NIS, TfOH | ||||
Comment |
MS 4A was included in the solvent. | ||||
| 1) 18+19, 2) NIS, TfOH | |||||
| COMMENT | |||||
| The alpha/beta ratio was determined by 1H NMR spectra. | |||||
| There are two phases in this reaction. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000021 | ||||
Issn |
Electronic | ||||
Doi |
10.1016/j.carres.2009.06.009 | ||||
PubMed ID |
19560125 | ||||
Journal Name |
Carbohydrate research. (2009) 344 (12): 1453-63. | ||||
Article Title |
Study on systematizing the synthesis of the a-series ganglioside glycans GT1a, GD1a, and GM1 using the newly developed N-Troc-protected GM3 and GalN intermediates. | ||||
Author |
Tatsuya, Komori; Akihiro, Imamura; Hiromune, Ando; Hideharu, Ishida; Makoto, Kiso | ||||
Affiliation |
Department of Applied Bioorganic Chemistry, Faculty of Applied Biological Sciences, Gifu University, 1-1 Yanagido, Gifu-shi, Gifu 501-1193, Japan. | ||||
Reference Id |
REF-0000-000022 | ||||
Source |
Carbohydrate Research, 344 (2009), 1453-1463 | ||||
Doi |
10.1016/j.carres.2009.06.009 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|