
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000513 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-000514

|
|||||||
Regist Date |
2012/06/21 16:10:12 | |||||||
| REACTANT | ||||||||
|
|
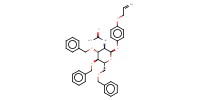
|
|
||||||
Mol |
0.62 mmol | |||||||
|
|
|
|||||||
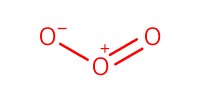
Reactant Type |
Ozone gas | |||||||
|
|
|
|||||||
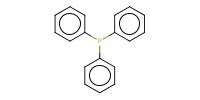
Mol |
1.9 mmol | |||||||
|
|
|
|||||||
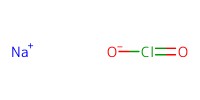
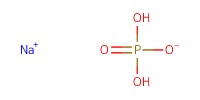
Reactant Type |
Sodium chlorite | |||||||
Mol |
6.2 mmol | |||||||
|
|
|
|||||||
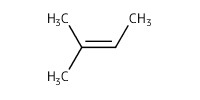
Mol |
0.74 mmol | |||||||
|
|
|
|||||||
Mol |
2.9 mmol | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
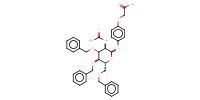
|
|
|||||
Yield |
70% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
2 hours, 2hours, 15 hours | |||||||
Reaction Temp |
-78 degree C, room temp, room temp | |||||||
Solvent |
CH2Cl2, CH2Cl2, t-buOH/H2O=10/2 | |||||||
Comment |
1) O3 gas, 2) Ph3P, 3) NaClO2, NaH2PO4, 2-methyl-2-butene, t-BuOH, H2O, 70% | |||||||
| COMMENT | ||||||||
| There are three phases in this reaction. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000016 | |||||||
Issn |
||||||||
PubMed ID |
19122320 | |||||||
Journal Name |
Chemical & pharmaceutical bulletin. (2009) 57 (1): 74-8. | |||||||
Article Title |
Syntheses and doxorubicin-inclusion abilities of beta-cyclodextrin derivatives with a hydroquinone alpha-glycoside residue attached at the primary side. | |||||||
Author |
Yoshiki, Oda; Masumi, Miura; Kenjiro, Hattori; Takashi, Yamanoi | |||||||
Affiliation |
The Noguchi Institute, Tokyo, Japan. | |||||||
Reference Id |
REF-0000-000017 | |||||||
Source |
CHEMICAL & PHARMACEUTICAL BULLETIN, Vol. 57 (2009), No. 1, 74 | |||||||
Doi |
10.1248/cpb.57.74 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|