
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000300 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000300

|
||||
Regist Date |
2012/06/21 15:17:45 | ||||
| REACTANT | |||||
|
|
|
||||
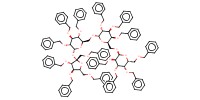
Reactant Type |
Fully benzylated sucrose-related oligosaccharide | ||||
Mol |
0.14 mmol | ||||
|
|

|
||||
Volume |
0.8 mL | ||||
|
|
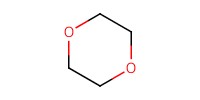
|
||||
Volume |
8 mL | ||||
| PRODUCT | |||||
MOLECULE ID |
|
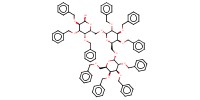
|
|||
Product Type |
Di- or trisaccharide unit | ||||
Yield |
92% | ||||
MOLECULE ID |
|
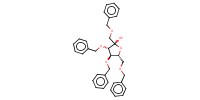
|
|||
Product Type |
Monosaccharide unit | ||||
Yield |
86% | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour | ||||
Reaction Temp |
room temp | ||||
Comment |
75% aq H2SO4 was used. | ||||
| COMMENT | |||||
| The alpha/beta ratio for compound 12 = 19/31. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000007 | ||||
Issn |
|||||
Doi |
10.1016/j.carres.2008.03.024 | ||||
PubMed ID |
18423585 | ||||
Journal Name |
Carbohydrate research. (2008) 343 (8): 1366-72. | ||||
Article Title |
Preparation of partially benzylated mono-, di-, and trisaccharides by selective cleavage of the beta-fructofuranosidic linkage in fully benzylated sucrose and sucrose-related oligosaccharides under acidic conditions. | ||||
Author |
Takashi, Yamanoi; Noriko, Misawa; Sho, Matsuda; Mikio, Watanabe | ||||
Affiliation |
The Noguchi Institute, Tokyo, Japan. tyama@noguchi.or.jp | ||||
Reference Id |
REF-0000-000008 | ||||
Source |
Carbohydrate Research 343 (2008) 1366‐1372 | ||||
Doi |
10.1016/j.carres.2008.03.024 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|