
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000262 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-000262

|
|||||||
Regist Date |
2012/06/21 15:07:59 | |||||||
| REACTANT | ||||||||
|
|
|
|||||||
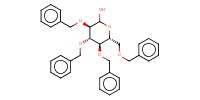
Reactant Type |
1-Hydroxy sugar | |||||||
Mol |
1 | |||||||
|
|
|
|||||||
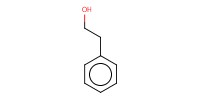
Reactant Type |
Alcohol | |||||||
Mol |
1 | |||||||
|
|

|
|||||||
Reactant Type |
Activator | |||||||
Mol |
0.05 | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
|
|
|||||
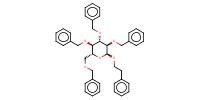
Product Type |
alpha | |||||||
Yield |
82%(alpha/beta=65/35) | |||||||
MOLECULE ID |
|
|
|
|||||
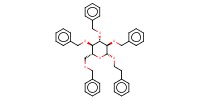
Product Type |
beta | |||||||
Yield |
82%(alpha/beta=65/35) | |||||||
MOLECULE ID |
|
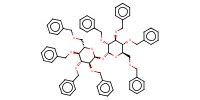
|
|
|||||
Product Type |
byproduct | |||||||
Yield |
11% | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
15 minutes | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
CH2Cl2 | |||||||
Catalyst |
Activator | |||||||
Comment |
anhydrous CaSO4 was included in the solvent. | |||||||
| Scheme 1 | ||||||||
| COMMENT | ||||||||
| The PMID could not be found. | ||||||||
| The alpha/beta ratio was determined by NMR. | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000012 | |||||||
Source |
HETEROCYCLES, Vol. 77, No. 1, 2009, pp. 445 - 460 | |||||||
Doi |
10.3987/COM-08-S(F)41 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|