
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000218 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-000218

|
|||||||
Regist Date |
2012/06/21 14:53:17 | |||||||
| REACTANT | ||||||||
|
|
|
|
||||||
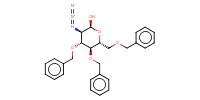
Reactant Type |
Acceptor | |||||||
Mol |
1 | |||||||
|
|
|
|||||||
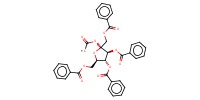
Reactant Type |
Donor[1,3,4,6-tetra-O-benzoyl-D-fructofuranosyl acetate (alpha/beta=3/1)] | |||||||
Mol |
1 | |||||||
|
|

|
|||||||
Reactant Type |
Activator | |||||||
Mol |
0.05 | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
|
||||||
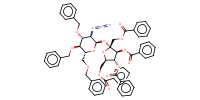
Product Type |
alpha+alpha | |||||||
Yield |
66%(alpha+alpha/alpha+beta/beta+alpha/beta/beta=14/5/67/14) | |||||||
MOLECULE ID |
|
|
||||||
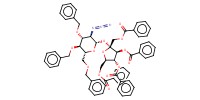
Product Type |
alpha+beta | |||||||
Yield |
66%(alpha+alpha/alpha+beta/beta+alpha/beta/beta=14/5/67/14) | |||||||
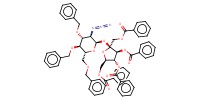
MOLECULE ID |
|
|
||||||
Product Type |
beta+alpha | |||||||
Yield |
66%(alpha+alpha/alpha+beta/beta+alpha/beta/beta=14/5/67/14) | |||||||
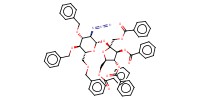
MOLECULE ID |
|
|
||||||
Product Type |
beta+beta | |||||||
Yield |
66%(alpha+alpha/alpha+beta/beta+alpha/beta/beta=14/5/67/14) | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
0.5 hour to overnight | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
CH2Cl2 | |||||||
Catalyst |
Activator | |||||||
Comment |
Anhydrous CaSO4 was included in the solvent. | |||||||
| COMMENT | ||||||||
| The PMID could not be found. | ||||||||
| CH2Cl2 was used as the solvent due to the poor solubility of aldopyranoses in PhCH3 | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000011 | |||||||
Source |
Tetrahedron Letters 48 (2007) 6458‐6462 | |||||||
Doi |
10.1016/j.tetlet.2007.07.092 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|