
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000201 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000201

|
||||
Regist Date |
2012/06/21 14:52:28 | ||||
| REACTANT | |||||
|
|
|
||||
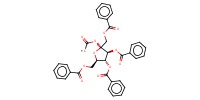
Reactant Type |
1,3,4,6-tetra-O-benzoyl-D-fructofuranosyl acetate (alpha/beta=3/1) | ||||
Mol |
1 | ||||
|
|

|
||||
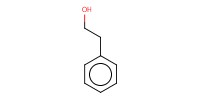
Reactant Type |
Lewis acid(activator) | ||||
Mol |
0.05 | ||||
|
|
|
||||
Mol |
1 | ||||
| PRODUCT | |||||
MOLECULE ID |
|
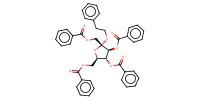
|
|||
Product Type |
alpha | ||||
Yield |
trace | ||||
MOLECULE ID |
|
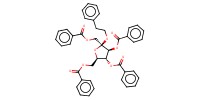
|
|||
Product Type |
beta | ||||
Yield |
trace | ||||
| REACTION DETAIL | |||||
Reaction Time |
0.5 to 2 hours | ||||
Reaction Temp |
0 degree C | ||||
Solvent |
PhCH3 | ||||
Catalyst |
activator | ||||
Comment |
Anhydrous CaSO4 was included in the solvent. | ||||
| COMMENT | |||||
| The PMID could not be found. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000011 | ||||
Source |
Tetrahedron Letters 48 (2007) 6458‐6462 | ||||
Doi |
10.1016/j.tetlet.2007.07.092 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|