
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000155 | |||||||
Submitter |
The Noguchi Institute | |||||||
Reaction ID |
R-0000-000155

|
|||||||
Regist Date |
2012/08/08 13:53:11 | |||||||
| REACTANT | ||||||||
|
|
|
|||||||
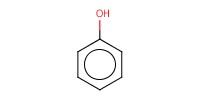
Reactant Type |
phenols | |||||||
Mol |
1 equiv. | |||||||
|
|
|
|||||||
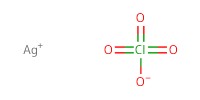
Reactant Type |
activator | |||||||
Mol |
1 equiv. | |||||||
|
|
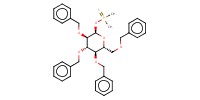
|
|
||||||
Mol |
1 equiv. | |||||||
| PRODUCT | ||||||||
MOLECULE ID |
|
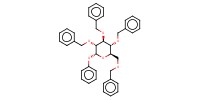
|
|
|||||
Product Type |
alpha | |||||||
Yield |
75%(alpha/beta=58/42) | |||||||
MOLECULE ID |
|
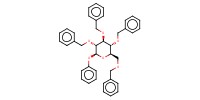
|
|
|||||
Product Type |
beta | |||||||
Yield |
75%(alpha/beta=58/42) | |||||||
| REACTION DETAIL | ||||||||
Reaction Time |
overnight | |||||||
Reaction Temp |
room temp | |||||||
Solvent |
benzene | |||||||
Catalyst |
AgClO4 | |||||||
Reaction Type |
aryl glycosidation | |||||||
Comment |
MS 4A was included in the solvent. | |||||||
| COMMENT | ||||||||
| The PMID could not be found. | ||||||||
| Keywords: glucopyranosyl dimethylphosphinothioate, phenolic compound, aryl glycoside, glycosylphenol, silver perchlorate, AgClO4 | ||||||||
| REFERENCE | ||||||||
Reference Id |
REF-0000-000010 | |||||||
Source |
Bulletin of the Chemical Society of Japan Vol. 67 (1994) , No. 5 pp.1488-1491 | |||||||
Doi |
10.1246/bcsj.67.1488 | |||||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|