
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000138 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000138

|
||||
Regist Date |
2012/06/21 14:30:48 | ||||
| REACTANT | |||||
|
|
|
||||
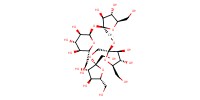
Reactant Type |
Starting material | ||||
Mol |
1 equiv. | ||||
|
|

|
||||
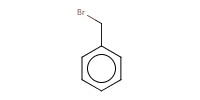
Mol |
56 equiv. | ||||
|
|
|
||||
Mol |
28 equiv. | ||||
| PRODUCT | |||||
MOLECULE ID |
|
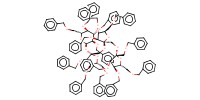
|
|||
Yield |
84% | ||||
| REACTION DETAIL | |||||
Reaction Time |
overnight | ||||
Reaction Temp |
room temp | ||||
Solvent |
DMF | ||||
Comment |
The reactants were mixed at 0 degree in Celsius before stirred at room temperature. | ||||
| COMMENT | |||||
| The alpha/beta ratio was NOT specified. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000007 | ||||
Issn |
|||||
Doi |
10.1016/j.carres.2008.03.024 | ||||
PubMed ID |
18423585 | ||||
Journal Name |
Carbohydrate research. (2008) 343 (8): 1366-72. | ||||
Article Title |
Preparation of partially benzylated mono-, di-, and trisaccharides by selective cleavage of the beta-fructofuranosidic linkage in fully benzylated sucrose and sucrose-related oligosaccharides under acidic conditions. | ||||
Author |
Takashi, Yamanoi; Noriko, Misawa; Sho, Matsuda; Mikio, Watanabe | ||||
Affiliation |
The Noguchi Institute, Tokyo, Japan. tyama@noguchi.or.jp | ||||
Reference Id |
REF-0000-000008 | ||||
Source |
Carbohydrate Research 343 (2008) 1366‐1372 | ||||
Doi |
10.1016/j.carres.2008.03.024 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|