
|
Glycan Syntheses |
JCGG ID |
JCGG-RAC0000060 | ||||
Submitter |
The Noguchi Institute | ||||
Reaction ID |
R-0000-000060

|
||||
Regist Date |
2012/06/21 13:01:09 | ||||
| REACTANT | |||||
|
|
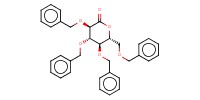
|
||||
Reactant Type |
Starting suger derivative | ||||
Mol |
0.54 mmol (1 equiv.) | ||||
|
|
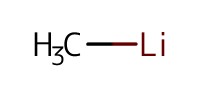
|
||||
Reactant Type |
MeLi | ||||
Mol |
0.64 mmol (1.2 equiv.) | ||||
|
|
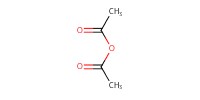
|
||||
Reactant Type |
Ac2O | ||||
Mol |
6 equiv. | ||||
Volume |
0.6 mL | ||||
| PRODUCT | |||||
MOLECULE ID |
|
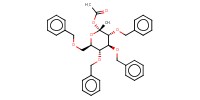
|
|||
Yield |
73% | ||||
| REACTION DETAIL | |||||
Reaction Time |
1 hour | ||||
| 1 hour | |||||
Reaction Temp |
-78 to -30 degree C | ||||
| -30 to 0 degree C | |||||
Solvent |
THF | ||||
| THF | |||||
Comment |
The addition of MeLi | ||||
| The addition of Ac2O | |||||
| Method B in Scheme 5 | |||||
| COMMENT | |||||
| The PMID could not be found. | |||||
| There are two phases in this reaction. | |||||
| MeLi can be substituted with MeMgBr. | |||||
| REFERENCE | |||||
Reference Id |
REF-0000-000004 | ||||
Source |
Tetrahedron 62 (2006) 10383‐10392 | ||||
Doi |
10.1016/j.tet.2006.08.059 | ||||
©2008-2013 Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
|