For measuring transport activity of sialin (silalic acid transporter), sialin is purified from insect cells and reconstituted into liposomes (Fig. 1). This enables us to analyze detailed transport function of sialin without artefacts by contaminating transporters. In addition, assay condition and driving force including pH gradient and membrane potential are precisely controlled by reconstituted system. These are large advantage to study function of transporter. |
| Category | Nucleotide sugar transporters |
| Protocol Name | Assay of lysosome sialic acid transporter |
Authors
 |
Miyaji, Takaaki
Department of Genomics and Proteomics, Advanced Science Research Center, Okayama University
Omote, Hiroshi
*
Department of Membrane Biochemistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University
Moriyama, Yoshinori
Department of Membrane Biochemistry, Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Buffer A
20 mM Tris-HCl (pH 8.0), 0.1 M sodium acetate, 10% glycerol, 0.5 mM dithiothreitol, 10 μg/mL pepstatin A and 10 μg/mL leupeptin |
| ● |
Solubilization buffer
20 mM MOPS-Tris (pH 7.0), 10% glycerol, 2% octylglucoside, 10 μg/mL pepstatin A and 10 μg/mL leupeptin |
| ● |
Wash buffer
20 mM MOPS-Tris (pH 7.0), 5 mM imidazole, 20% glycerol and 1% octylglucoside |
| ● |
Reconstitution buffer
20 mM MOPS-Tris (pH 7.0), 0.1 M potassium acetate and 5 mM magnesium acetate, 0.5 mM dithiothreitol |
| ● |
Reaction mixture
20 mM MOPS-Tris (pH 7.0) or 40 mM MES (pH 5.6), 5 mM magnesium acetate, 4 mM KCl, 0.1 M potassium acetate |
|
Instruments
 |
|
| Methods |
|
1. |
|
| 1) |
Suspend infected insect cells (1–2 × 108 cells) by buffer A and disrupt cells by sonication using a TOMY UD200 tip sonifier. |
Comment 0
|

|
| 2) |
Centrifuge cell lysates at 700 × g for 10 min to remove debris. |
Comment 0
|

|
| 3) |
Centrifuge supernatant at 160,000 × g for 1 h. |
Comment 0
|

|
| 4) |
Suspend the pellet (membrane fraction) in the solubilization buffer to give approximately 2 mg protein/mL. |
Comment 0
|

|
| 5) |
Centrifuge at 260,000 × g for 30 min to remove insolubilzed membrane. |
Comment 0
|

|
| 6) |
Add supernatant to 1 mL of Ni-NTA Superflow resin (Qiagen) and incubate for 4 h. |
Comment 0
|

|
| 7) |
Wash resin with 20 mL of wash buffer in a column. |
Comment 0
|

|
| 8) |
Elute sialin from the resin with 3 mL of the same buffer containing 60 mM imidazole. |
Comment 0
|

|
| 9) |
Store eluate containing purified sialin at −80°C. Purified sialin is stable without loss of activity for at least a few months. |
Comment 0
|
|
|
|
2. |
Reconstitution & Transport assay |
| 1) |
Mix 20 μg of sialin with liposomes (0.5 mg lipid*; see Comment), freeze at −80°C and leave at this temperature for more than 10 min. |
Comment 1
|

|
| 2) |
Quickly thaw mixture by holding the sample tube in the hands and dilute 30-fold with reconstitution buffer. |
Comment 0
|

|
| 3) |
Sediment proteoliposomes by centrifugation at 200,000 × g for 1 h at 4°C and suspend in 0.2 mL of reconstitution buffer. |
Comment 0
|

|
| 4) |
Add proteoliposomes (0.5 μg total protein) into the reaction mixture containing 100 μM [6-3H] sialic acid (0.5 MBq/mmol) and incubate at 27°C. |
Comment 0
|

|
| 5) |
Withdraw 120 μL aliquots at the appropriate times and centrifuge through a Sephadex G-50 (fine) spin column at 760 × g for 2 min. |
Comment 0
|

|
| 6) |
Measure radioactivity in the eluate. |
Comment 0
|
|
|
| Notes | Purification
All procedures should be carried out under 4°C.
Reconstitution & Transport assay
Reconstitution of purified recombinant sialin into liposomes is carried out by the freeze-thaw method. |
| Figure & Legends |
Figure & Legends 
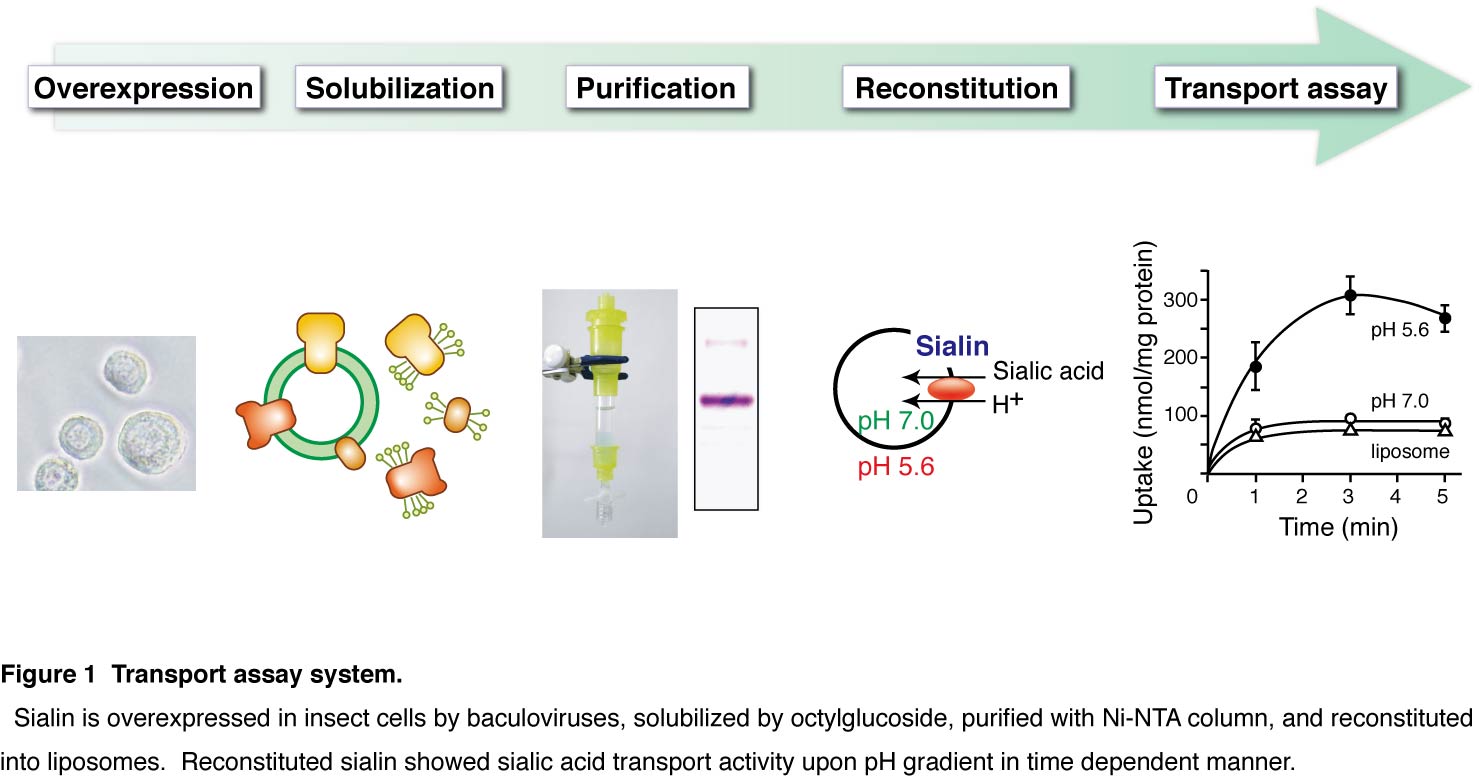
|
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2015-12-17 11:18:06 |
- Miyaji, T., Echigo, N., Hiasa, M., Senoh, S., Omote, H., and Moriyama Y. (2008) Identification of a vesicular aspartate transporter. Proc. Natl. Acad. Sci. USA. 105, 11720–11724 [PMID : 18695252]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Miyaji, Takaaki,
Omote, Hiroshi,
Moriyama, Yoshinori,
(2015). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.26,4,2024 .
How to Cite this Work in Website:
Miyaji, Takaaki,
Omote, Hiroshi,
Moriyama, Yoshinori,
(2015).
Assay of lysosome sialic acid transporter.
Retrieved 26,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t93.
html source
Miyaji, Takaaki,
Omote, Hiroshi,
Moriyama, Yoshinori,
(2015).
<b>Assay of lysosome sialic acid transporter</b>.
Retrieved 4 26,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t93" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t93</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|