Influenza virus hemagglutinin (HA) attaches to sialoglycoconjugates on the host cell surface to initiate infection. Binding specificity of influenza virus for sialooligosaccharide moieties on the cell surface is a critical factor for acquiring transmission ability to differentiate host species (Suzuki Y. et al. 1992; Suzuki T. et al. 2001; Shinya K et al. 2005; Takemae N. et al. 2009; Sriwilaijaroen N. et al. 2009). TLC-based method for assay of receptor binding specificity of influenza viruses using sialoglycosphingolipids (gangliosides) (Suzuki Y. et al. 1992; Suzuki T. et al. 2001; Shinya K et al. 2005) as viral receptors is described in this paper. |
| Category | Sugar binding proteins |
| Protocol Name | Thin-layer chromatography (TLC) -based assay for determination of influenza virus-host receptor binding specificity |
Authors
 |
Suzuki, Yasuo
*
Health Science Hills, College of Life and Health Sciences, Chubu University
Sriwilaijaroen, Nongluk
(1) Health Science Hills, College of Life and Health Sciences, Chubu University, (2) Faculty of Medicine, Thammasat University (Rangsit Campus)
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Influenza viruses: Influenza A, B, and C viruses can be propagated in cell culture (e.g. Madin-Darby canine kidney (MDCK), human airway epithelial (A549) cells) or in embryonic egg-based culture (e.g. amniotic, allantoic cavities of 9-day-old to 11-day-old chicken eggs), depending on virus types used and the purpose of the study, concentrated by high-speed centrifugation or purified by sucrose density gradient centrifugation and stored at −80°C. Just before use, virus is suspended in phosphate buffered saline (PBS) at the concentration of 28 hemagglutination units (HAU) and kept on ice for no longer than 1 h. |
| ● |
Gangliosides: Native gangliosides can be isolated from a wide variety of tissues related to predominant ganglioside species present in. Ganglio-series gangliosides, such as GM1a, GM1b, GD1a, GD1b, GT1a, GT1b, and GQ1b are mainly found in and thus taken from brains of mammals including bovine, pig and horse by an anion exchange and Iatrobeads column chromatography. Ganglioside GM3(Neu5Ac) [II3(Neu5Ac)LacCer] and GM3(Neu5Gc) [II3(Neu5Gc)LacCer] are prepared from human liver and equine erythrocytes, respectively. GT3(Neu5Ac), GD3(Neu5Ac), and GD2(Neu5Ac) are obtained from bovine butter milk and brain, respectively. GD3(Neu5,9Ac) is from bovine milk. GM4 is from chicken liver. Lacto-series gangliosides containing type II sugar chain, sialylparagloboside [IV3(Neu5Ac)nLc4Cer], IV3(Neu5Ac)nLc6Cer, and VIII3(Neu5Ac)VI3(Neu5Ac)IV6kladoLc8Cer with branched sugar chain are from human erythrocytes. IV3(Neu5Gc)nLc4Cer is from bovine erythrocytes and IV6(Neu5Ac)nLc4Cer is from human meconium. |
| ● |
Developing solvent system I: Chloroform/methanol/12 mM MgCl2 (5:4:1, v/v/v) |
| ● |
Developing solvent system II: Chloroform/methanol/2.5 M ammonia (50:40:9, v/v/v) |
| ● |
Phosphate buffered saline (PBS): 1.5 mM KH2PO4, 14 mM Na2HPO4, 2.7 mM KCl and 131 mM NaCl adjusted to pH 7.2 |
| ● |
Blocking solution: 1 g of egg albumin (crystallized) and 1 g of polyvinylpyrrolidone (PVP) in 100 mL of PBS |
| ● |
Antibodies: Monoclonal antibodies directed for HA of influenza virus A and B viruses and for hemagglutinin-esterase (HE) of influenza C virus can be used. Specific polyclonal antibodies directed for influenza A, B and C virus can also be used. Mouse monoclonal antibody, 1A (IgG), directed to the HA of A/PR/8/34 (H1N1) strain was prepared as follows: A Balb/c mouse was immunized with 1000 HAU of purified A/PR/8/34 virions intraperitineally. Two months later, the mouse was given a similar intravenous booster injection. Four days after the second injection, the spleen cells of the mouse were fused with myeloma cells X63-Ag8-6.3.5. The culture fluids from the fused cells were screened by ELISA, hemagglutination, and sialidase inhibition tests with the homologous influenza viruses for the detection of antibodies. A hybridoma cell line producing antibody against HA of A/PR/8/34 was cloned in soft agar and named 1A antibody. Ascites fluid was collected 7 to 10 days after the intraperitoneal injection of hybridoma cells into pristane-treated mouse. Secondary antibody: horseradish peroxidase (HRP)-conjugated secondary antibody directed against the host species of the primary antibody. HRP-conjugated goat anti-mouse IgG antibody may be used as appropriate for the anti-HA mouse monoclonal antibody. |
| ● |
Substrate solution: Immediately prior to use, 10 mM Tris-HCl buffer (pH 7.2), 3% 4-chloro-1-naphthol in methanol (stored at 4°C) and 3% aqueous hydrogen peroxide (H2O2) (stored at 4°C) are mixed at the ratio of 5:1:0.02, v/v/v. |
|
Instruments
 |
| ● |
TLC Developing chamber lined with a filter paper |
| ● |
Covered plastic rectangular container: The size of the container may be different depending on the size of TLC plate |
| ● |
|
| ● |
|
| ● |
|
|
| Methods |
|
1. |
Thin-layer chromatography (TLC) -based assay for determination of influenza virus-host receptor binding specificity |
| 1) |
Apply gangliosides (10–1000 pmol) on a silica gel Polygram Sil G plate (Nagel, Germany, usually 5 cm × 1 cm for 1 lane) using a microsyringe. |
Comment 0
|

|
| 2) |
Develop the plate to near the top of the plate in the solvent system of chloroform/methanol/12mM MgCl2 (5:4:1, v/v/v, solvent system I) or chloroform/methanol/2.5M ammonia (50:40:9, v/v/v, solvent system II). Solvent system II is recommended for separation of Neu5Acα2-3Gal- from Neu5α2-6Gal-containing ganglioside and for separation of Neu5Gc- from Neu5Ac-containing ganglioside. |
Comment 0
|

|
| 4) |
Put the plate into a covered plastic rectangular container and block with 1% egg albumin and 1% PVP in PBS (blocking solution; 0.1 mL/cm2 of the bottom of the box) at room temperature for 2 h. |
Comment 0
|

|
| 5) |
Remove the blocking solution by suction and wash the plate with PBS (0.2–0.3 mL/cm2 of the bottom) three times. |
Comment 0
|

|
| 6) |
Incubate the plate with virus suspension in PBS (0.1 mL/cm2 of the bottom) for a maximum of 12 h at 4°C with gentle shaking. In this condition, viral sialidase activity of influenza A and B viruses and 9-O-acetyl-Neu5Ac acetylesterase activity of influenza C virus are inactive. |
Comment 0
|

|
| 7) |
Following five washes with PBS (0.2–0.3 mL/cm2 of the bottom), block the plate with blocking solution (0.1 mL/cm2 of the bottom) at 4°C for 2 h. |
Comment 0
|

|
| 8) |
Wash the plate with PBS three times (0.2–0.3 mL/cm2 of the bottom) before incubation with antibody (anti-HA mouse monoclonal antibody for A/PR/8/34) in the blocking solution (0.1 mL/cm2 of the bottom) at 4°C for 2 h. |
Comment 0
|

|
| 9) |
After washing with PBS five times (0.2–0.3 mL/cm2 of the bottom), block the plate with the blocking solution (0.1 mL/cm2 of the bottom) at 4°C for 2 h. |
Comment 0
|

|
| 10) |
Following removal of the blocking solution and washing the plate with PBS three times (0.2–0.3 mL/cm2 of the bottom), anti-HA antibody is reacted at 4°C for 2 h with HRP-conjugated goat anti-mouse IgG (light and heavy chain) for A/PR/8/34. |
Comment 0
|

|
| 11) |
After washing with PBS five times, immerse the plate in the peroxidase substrate solution at room temperature for 20 min. |
Comment 0
|

|
| 12) |
Determine binding reactivity of the virus to each ganglioside by scanning the stained chromatogram at 578 nm with 780 nm as reference. |
Comment 0
|
|
|
| Notes | Influenza viruses should be handled in the facility of Biological Safety Level 2 or 3. Therefore, handling of the viruses should be under the control of national law. |
| Figure & Legends |
Figure & Legends 
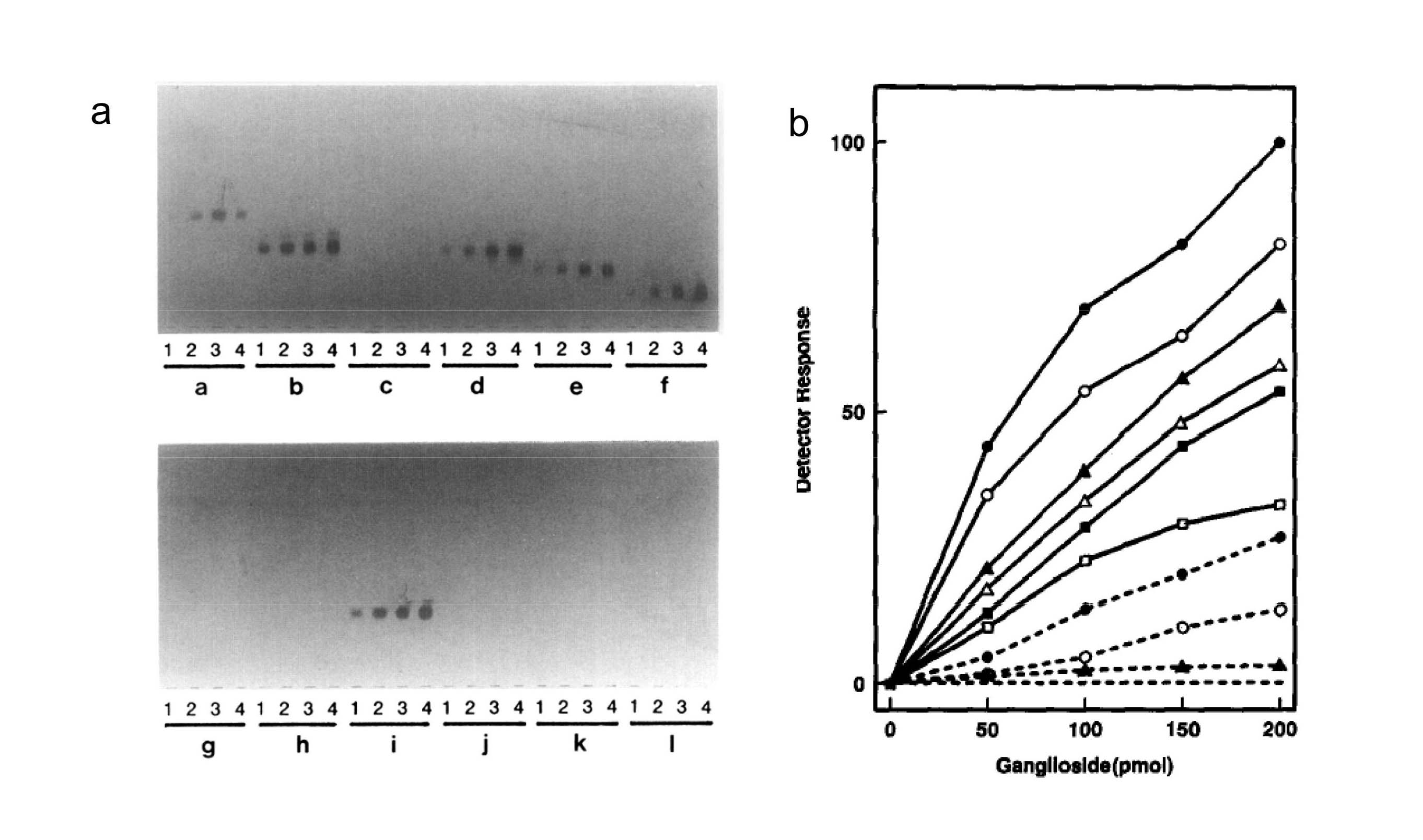
Fig. 1. Example of binding activity of gangliosides to laboratory strain, A/PR8/8/34 (H1N1)
(a) Various amounts of each gangliosides, 50 pmol (lane 1), 100 pmol (lane 2), 150 pmol (lane 3) and 200 pmol (lane 4) were developed in TLC plates (95 × 195 mm) (Nagel Sil G). The plates were incubated with the virus of 28 HAU at 4°C for 9 h, and then stained by TLC/virus-binding assay as described in the Methods. The inner geometry of the container was 100 (L) × 200 (W) × 30mm (H) for the bottom and 106 (L) × 206 (W) × 10 mm (H) for the cover. Group a, GM3; group b, IV3(Neu5Ac)Lc4Cer; group c, IV6(Neu5Ac)nLc4Cer; group d, GM1b; group e, GD1a; group f, GT1b; group g, GM3 (Neu5Gc); group h, GM2; group i, IV3 (Neu5Gc)nLc4Cer; group j, IV3(Neu5Gc)nLc4Cer; group k, GM1a; and group l, GD1b.
(b) Binding activity of the virus to each ganglioside was determined by scanning the positive spots with a dual wavelength chromatoscanner. (―●―), IV3(Neu5Ac)Lc4Cer; (―○―), IV3(Neu5Ac)nLc4Cer; (―△―), IV3(Neu5Ac)nLc6Cer; (―■―), GM1b; (―□―), GD1a; (-----●----), GT1b; (----○----), GM3(Neu5Ac); (----▲----), IV6(Neu5Ac)Lc4Cer and IV6(Neu5Ac)nLc4Cer; (--------), GM4(Neu5Ac), GM3(Neu5Gc), GM3(Neu4Ac, 5Gc), GM2, GM1a, IV3(Neu5Gc)nLc4Cer, GD2(Neu5Ac), GD3(Neu5Ac), GD3(Neu5Gc), GD3(Neu5,9Ac), GD1b, GT1b, GQ1b, and GT3(Neu5Ac).
Reprinted from Virology, 189(1), Suzuki Y. et al., Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains, 121–31, 1992, with permission from Elsevier. doi:10.1016/0042-6822(92)90687-K.

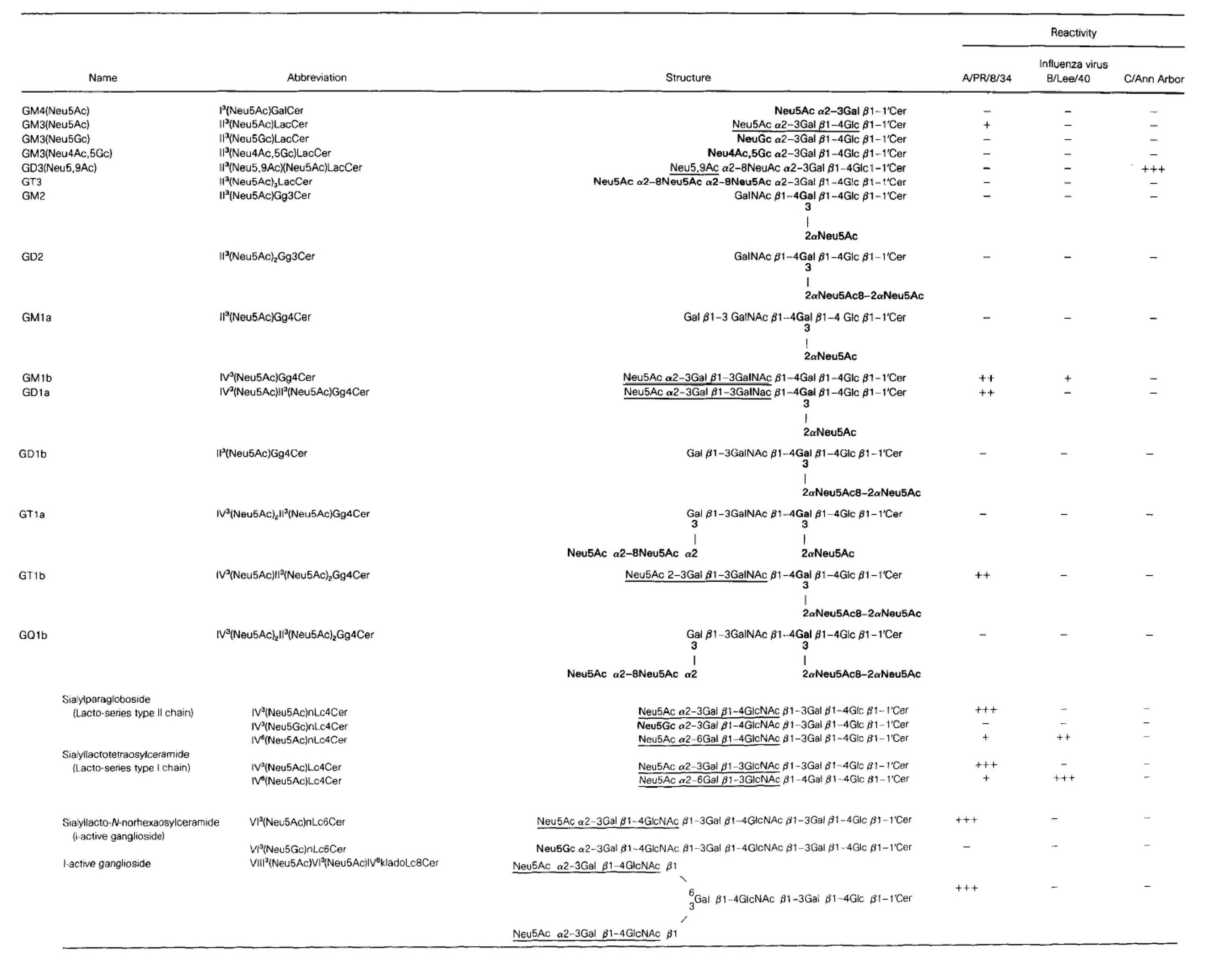
Table 1. Strain-specific binding reactivity of gangliosides to human influenza A, B and C viruses
Note: Gangliosides (each 1 nmol) were developed on TLC plates (Nagel Sil G) in the solvent system I. The plates were then incubated with each of the influenza viruses, A/PR/8/34 (H1N1), B/Lee/40, and C/Ann Arbor of 28 HAU at 4°C for 9h and stained by TLC/virus –binding assay as described in this method. Reactivity was expressed as (+++) for high binding, (++) for moderate binding, and (+) for low binding. (-) shows negative binding. Sugar epitopes involved in the virus binding were indicated with underline. Sialyl residues not recognized by the A and B viruses were indicated with boldface. Structure of ganglioside is available in “LipidBank”, The official database of Japanese Conference on the Biochemistry of Lipids (JCBL, http://lipidbank.jp/).
Reprinted from Virology, 189(1), Suzuki Y. et al., Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains, 121–31, 1992, with permission from Elsevier. doi:10.1016/0042-6822(92)90687-K. |
| Copyrights |
Copyright 1992. Elsevier, for Fig.1 & Table 1 in Figure & Legends
Copyright 2010. Ritsumeikan University, JCGGDB & AIST. for the rest of the contents |
| Date of registration:2014-10-20 16:42:24 |
- Suzuki, Y., Nakao, T., Ito, T., Watanabe, N., Toda, Y., Xu, G., Suzuki, T., Kobayashi, T., Kimura, Y., and Yamada, A. (1992) Structural determination of gangliosides that bind to influenza A, B, and C viruses by an improved binding assay: strain-specific receptor epitopes in sialo-sugar chains. Virology, 189, 121–31. [PMID : 1376537]
- Suzuki, T., Portner, A., Scroggs, R.A., Uchikawa, M., Koyama, N., Matsuo, K., Suzuki, Y., and Takimoto, T. (2001) Receptor specificities of human respiroviruses. J. Virol. 75, 4604–4613. [PMID : 11312330]
- Shinya, K., Hatta, M., Yamada, S., Takada, A., Watanabe, S., Halfmann, P., Horimoto, T., Neumann, G., Kim, J.H., Lim, W., Guan, Y., Peiris, M., Kiso, M., Suzuki, T., Suzuki, Y., and Kawaoka, Y. (2005) Characterization of a Human H5N1 Influenza A Virus Isolated in 2003. J. Virol. 79, 9926–9932. [PMID : 16014953]
- Takemae, N., Ruttanapumma, R., Parchariyanon, S., Yoneyama, S., Hayashi, T., Hiramatsu, H., Sriwilaijaroen, N., Uchida, Y., Kondo, S., Yagi, H., Kato, K., Suzuki, Y., and Saito, T. (2010) Alterations in receptor binding properties of swine influenza viruses of H1 subtype after isolation in embryonated chicken eggs. J. gen. Virol. 91, 938–48. [PMID : 20007353]
- Sriwilaijaroen, N., Kondo, S., Yagi, H., Wilairat, P., Hiramatsu H., Ito, M., Ito, M., Kato, K., and Suzukim Y. (2009) Analysis of N-glycans in embryonated chicken egg chorioallantoic and amniotic cells responsible for binding and adaptation of influenza viruses. Glycoconjugate J. 26, 433–443. [PMID : 18853253]
|
|
For those who wish to reuse the work, please contact JCGGDB management office (jcggdb-ml@aist.go.jp).
|
|