Chondroitin sulfate (CS) is usually found attached to a core protein as part of a proteoglycan (PG). Some CS-PGs are an important structural component of connective tissue matrix (such as skin and cartilage) and provide much of its resistance to compression. Others are found on cell surfaces and function mainly as co-receptors. CS biosynthesis (Fig. 1) is initiated by the transfer of a GalNAc residue to the tetrasaccharide linkage region GlcAβ1-3Galβ1-3Galβ1-4Xyl attached to specific serine residues of the core protein. Once the first GalNAc is transferred, the elongation process is started by the alternate addition of GlcA and GalNAc. To date, six CS biosynthetic enzymes have been identified (Fig. 1). These enzyme proteins can form a complex in various combinations, resulting in a marked augmentation of glycosyltransferase activities and the expression of the chondroitin polymerization activities. Therefore, biosynthetic processes of chondroitin are achieved by multiple combinations of the different enzymes and each combination may play a unique role in the biosynthesis of chondroitin. |
| Category | Glycosyltransferases & related proteins |
| Protocol Name | Enzyme assay of GAG glycosyltransferases for chondroitin sulfate |
Authors
 |
Nadanaka, Satomi
Department of Biochemistry, Kobe Pharmaceutical University
Kitagawa, Hiroshi
*
Department of Biochemistry, Kobe Pharmaceutical University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
GlcAT-I activities
Substrate: Gal β1-3Gal β1-4Xyl*
Donor: UDP-[U - 14C]GlcA (285.2 mCi/mmol) (PerkinElmer, Waltham, MA)
* Gal β1-3Gal β1-4Xyl, an acceptor substrate for GlcAT-I, was a gift from Dr. Nancy B. Schwartz (University Chicago). |
| ● |
CS-GlcAT-II activities
Substrate: polymer chondroitin (Seikagaku Corporation, Tokyo, Japan, Cat No. 400640)
Donor: UDP-[U - 14C]GlcA (285.2 mCi/mmol) (PerkinElmer) |
| ● |
GalNAcT-II activities
Substrate: polymer chondroitin (Seikagaku Corporation, Cat No. 400640)
Donor: UDP-[3H]GalNAc (10 Ci/mmol) (PerkinElmer) |
| ● |
Polymerization activities
Substrate: GlcAβ1-3Galβ1-O-C2H4NH-benzyloxycarbonyl (chemically-synthesized linkage region analog) or
GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-O-Ser** (chemically-synthesized tetrasaccharide serine)
Donor: UDP-[3H]GalNAc (10 Ci/mmol) and UDP-[U - 14C]GlcA (285.2 mCi/mmol) (PerkinElmer)
** Acceptor substrates for the polymerization reaction, GlcAβ1-3Galβ1-O-C2H4NH-benzyloxycarbonyl and GlcAβ1-3Galβ1-3Galβ1-4Xylβ1-O-Ser, were kindly provided from Prof. Jun-ichi Tamura (Tottori University). |
|
Instruments
 |
| ● |
GlcAT-I activities
Chip column packed with Dowex 1-X8 (PO42- form)
2300TR liquid scintillation counter (PerkinElmer) |
| ● |
GalNAcT-II activities and CS-GlcAT-II activities
Syringe column (TERUMO SS-01T) packed with Superdex G-25 superfine (GE Healthcare, Little Chalfont, UK)1)
2300TR liquid scintillation counter (PerkinElmer) |
| ● |
Polymerization activities
Superdex Peptide HR 10/30 or Superdex 200 10/300 GL (GE Healthcare)
FPLC
2300TR liquid scintillation counter (PerkinElmer) |
|
| Methods |
|
1. |
Enzyme assay of GAG glycosyltransferases for chondroitin sulfate |
| 1) |
The following reaction mixtures of a total volume of 30 μL are prepared.
GlcAT-I activities
10 μL of enzyme source (see Comment *)
50 mM MES-NaOH (pH 6.5)
2 mM MnCl2
Gal β1-3Gal β1-4Xyl (1 nmol)
14.3 μM UDP-[14C]GlcA (approx. 1.46 × 105 dpm)
CS-GlcAT-II activities
10 μL of enzyme source
50 mM MES-NaOH (pH 6.5)
10 mM MnCl2
Chondroitin (171 μg)
14.3 μM UDP-[14C]GlcA (approx. 1.46 × 105 dpm)
GalNAcT-II activities
10 μL of enzyme source
50 mM MES-NaOH (pH 6.5)
10 mM MnCl2
171 μM ATP-Na
Chondroitin (171 μg)
8.57 μM UDP-[3H]GalNAc (approx. 3.60 × 105 dpm)
Polymerization activities
10 μL of enzyme source (Co-expression of at least two enzyme proteins is required for the polymerization reaction)
100 mM MES-NaOH (pH 6.5)
10 mM MnCl2
GlcAβ1-3Galβ1-O-C2H4NH-benzyloxycarbonyl (100 nmol) (see Comment **)
250 μM UDP-[3H]GalNAc (approx. 50.0 × 105 dpm)
250 μM UDP-GlcA |
Comment 1
|

|
| 2) |
Reaction mixtures of GlcAT-I, CS-GlcAT-II, and GalNAcT-II are incubated at 37˚C for 2 h.
Polymerization reaction is performed at 37˚C for 12 h. |
Comment 0
|

|
| 3) |
Each enzyme activity is measured according to the following method.
GlcAT-I activities (Fig. 2)
i) Preparation of chip columns 2)
Pipet chip (1 mL)
↓ pack with Dowex 1-X8 equilibrated with 5 mM phosphate buffer (pH 6.8).
Dowex 1-X8 (PO42- form)
ii) Assay
GlcAT-I reaction products
↓ add 1 mL of 5 mM phosphate buffer (pH 6.8).
↓ apply to a chip column.
↓ wash a chip column with 1 mL of 5 mM phosphate buffer (pH 6.8).
Flow-through and wash fractions (2 mL)
↓ analyze the radioactivity of the effluent fractions.
GalNAcT-II and CS-GlcAT-II activities (Fig. 3) 3)
i) Preparation of syringe columns
Syringe columns (TERUMO SS-01T)
↓ pack with Sephadex G-25 (superfine) equilibrated with 0.2 M NH4HCO3.
↓ centrifuge at 800 rpm for 5 min.
↓ centrifuge again at 2,000 rpm for 5 min.
ii) Assay
Samples (30 μL)
↓ add 20 μL of 0.2 M NH4HCO3.
Samples (50 μL)
↓ apply to the packed syringe column.
↓ centrifuge at 2,000 rpm for 5 min.
↓ add 50 μL of 0.2 M NH4HCO3 to the top of the syringe columns.
↓ centrifuge at 2,000 rpm for 5 min.
Eluates
↓ measure by scintillation counter.
Polymerization activities (Fig. 4) 4)
The reaction products
↓ analyze using a Superdex Peptide HR 10/30 column (see Comment *).
↓ measure radioactivity using a liquid scintillation counter. |
Comment 1
|

|
| 4) |
Each reaction product is confirmed.
GlcAT-I-reaction products
GlcAT-I reaction products
↓ pool and evaporate.
GlcAT-I reaction products
↓ digest with 22 mIU of β-glucuronidase (EC 4.2.2.5, Prozyme Inc. Code No. GKGAG-5007)
in a total volume of 30 μL of 50 mM sodium citrate buffer (pH 4.5) at 37˚C overnight.
↓ analyze digested or undigested samples on a Asahipak GS-320 column (GL Science Inc., Tokyo, Japan).
The radioactivity of the GlcAT-I-reaction products is released by digestion with β-glucuronidase and
eluted at the free [14C]GlcA position.
CS-GlcAT-II and GalNAcT-II-reaction products
CS-GlcAT-II or GalNAcT-II reaction products
↓ pool and evaporate.
CS-GlcAT-II or GalNAcT-II reaction products (100-200 μg)
↓ digest with 100 mIU of chondroitinase AC-II (Seikagaku Corporation) in a total volume of 30 μL of 50 mM
sodium acetate buffer (pH 6.0) at 37˚C overnight.
↓ analyze by a Superdex peptide HR 10/30 column.
The radioactivity peak of the CS-GlcAT-II and GalNAcT-II-reaction products eluted near the void volume
is subjected to digestion with chondroitinase AC-II and shifted to [14C]GlcAβ1-3GalNAc and free [3H]GalNAc
position, respectively.
Polymerized products
Polymerized products (Fig. 4)
↓ pool and evaporate.
Polymerized products
↓ digest with 100 mIU of chondroitinase AC-II (Seikagaku Corporation) in a total volume of 30 μL of 50 mM
sodium acetate buffer (pH 6.0) at 37˚C overnight.
↓ analyze using a Superdex peptide HR 10/30 column.
The radioactivity peak of the polymerized products eluted near the void volume is degraded by digestion
with chondroitinase AC-II and shifted to the elution position of the unsaturated disaccharide. |
Comment 0
|
|
|
| Figure & Legends |
Figure & Legends 
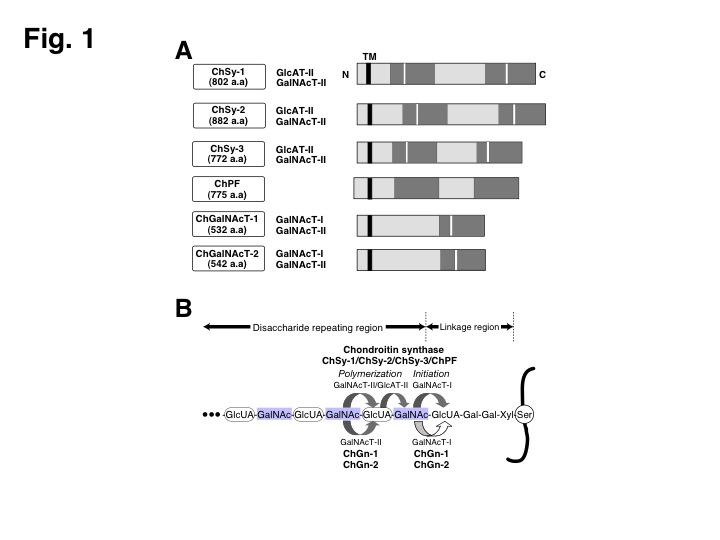
Fig. 1. The six cloned members involved in the CS biosynthesis.
(A) Schematic structures of six genes are shown. Highly conserved regions are indicated by gray bars. White bars show DXD motif. a.a., amino acid; TM, transmembrane domain. In addition, glycosyltransferase activities detected in each gene product are summarized. (B) The biosynthesis of CS in mammals. Mammalian CS is biosynthesized by multiple combinations of at least two proteins out of ChSy-1, ChSy-2, ChSy-3, and ChPF. It has been reported that ChGalNAcT-I and ChGalNAcT-II control the number of CS chains attached to a core protein and the length of CS chains, respectively.

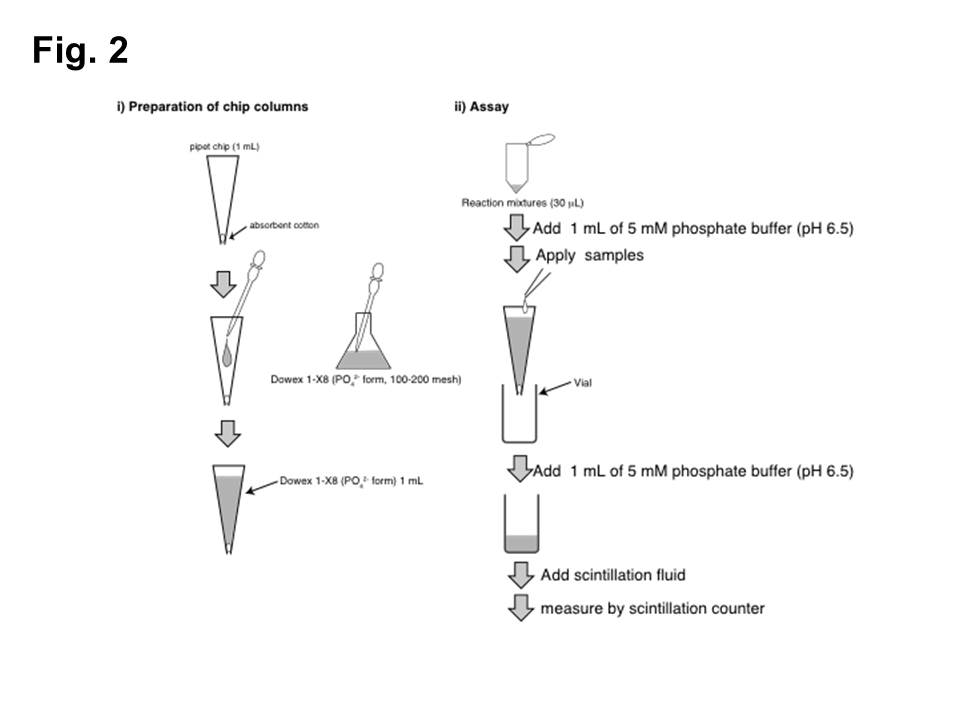
Fig. 2. Measurement of GlcAT-I activities by chip column assay.

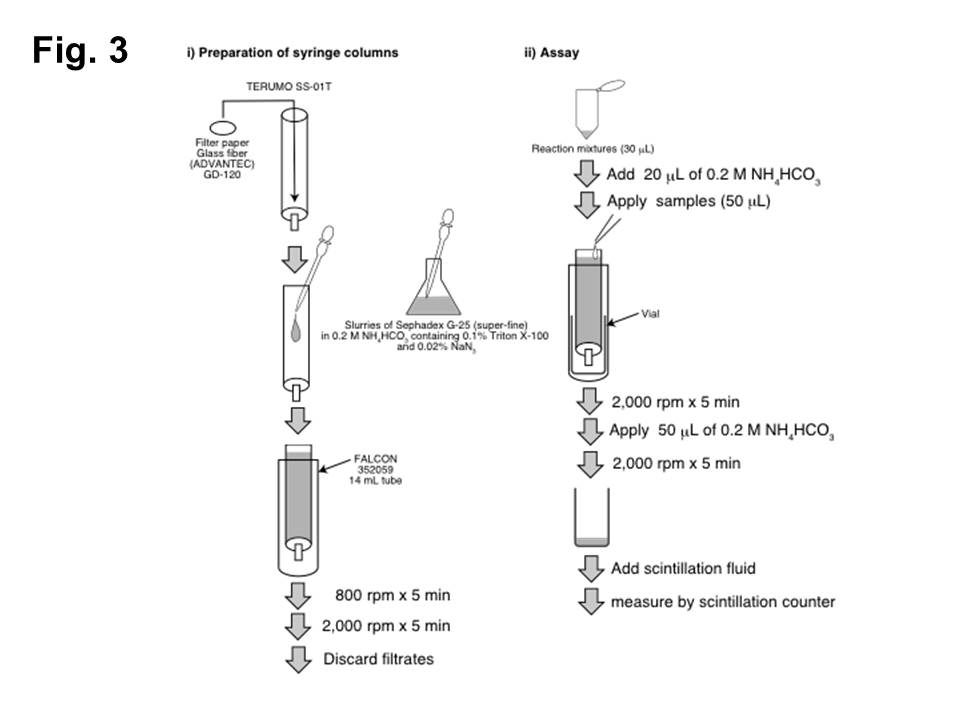
Fig. 3. GalNAcT-II and CS-GlcAT-II assays using a syringe column.

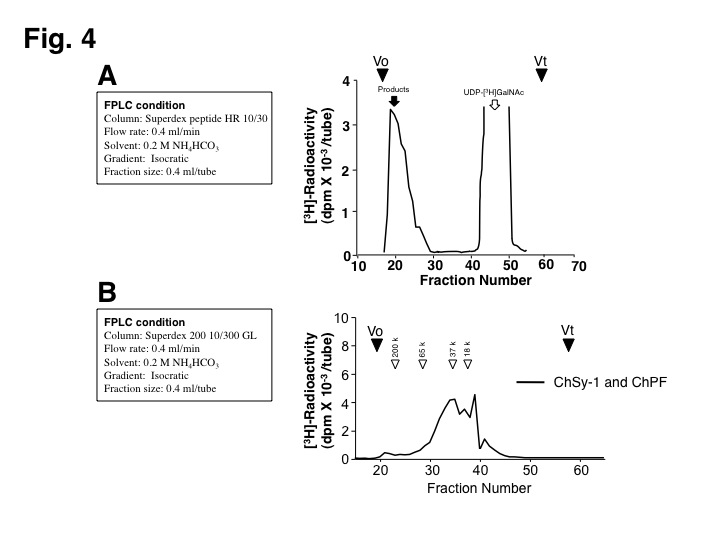
Fig. 4. Analysis of polymerized CS chains.
(A) The polymerization reaction products were analyzed on a Superdex peptide column.
(B) Molecular size of CS chains formed by the different combination of enzymes was determined by gel filtration chromatography on a Superdex 200 column. White arrowheads indicate the elution positions of commercial dextrans of known molecular weights. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-09-11 16:10:06 |
- Kitagawa, H., Tsuchida, K., Ujikawa, M., and Sugahara, K. (1995) Detection and characterization of UDP-GalNAc: chondroitin N-acetylgalactosaminyltransferase in bovine serum using a simple assay method. J Biochem. 117, 1083–1087 [PMID : 8586623]
- Weinstein, J., de Souza-e-Silva, U., and Paulson, J. C. (1982) Sialylation of glycoprotein oligosaccharides N-linked to asparagine. Enzymatic characterization of a Gal beta 1 to 3(4)GlcNAc alpha 2 to 3 sialyltransferase and a Gal beta 1 to 4GlcNAc alpha 2 to 6 sialyltransferase from rat liver. J Biol Chem. 257, 13845–13853 [PMID : 7142180]
- Tsuchida, K., Lind, T., Kitagawa, H., Lindahl, U., Sugahara, K., and Lidholt, K. (1999) Purification and characterization of fetal bovine serum beta-N-acetyl-D-galactosaminyltransferase and beta-D-glucuronyltransferase involved in chondroitin sulfate biosynthesis. Eur J Biochem. 264, 461–467 [PMID : 10491092]
- Kitagawa, H., Izumikawa, T., Uyama, T., and Sugahara, K. (2003) Molecular cloning of a chondroitin polymerizing factor that cooperates with chondroitin synthase for chondroitin polymerization. J Biol Chem. 278, 23666–23671 [PMID : 12716890]
- Kitagawa, H., Ujikawa, M., and Sugahara, K. (1996) Developmental changes in serum UDP-GlcA: chondroitin glucuronyltransferase activity. J Biol Chem. 271, 6583–6585 [PMID : 8636071]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Nadanaka, Satomi,
Kitagawa, Hiroshi,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.25,4,2024 .
How to Cite this Work in Website:
Nadanaka, Satomi,
Kitagawa, Hiroshi,
(2014).
Enzyme assay of GAG glycosyltransferases for chondroitin sulfate.
Retrieved 25,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t82.
html source
Nadanaka, Satomi,
Kitagawa, Hiroshi,
(2014).
<b>Enzyme assay of GAG glycosyltransferases for chondroitin sulfate</b>.
Retrieved 4 25,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t82" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t82</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|