Lectins are carbohydrate binding proteins, and can be used to discriminate and analyze the glycan structures of glycoproteins. The lectin blotting technique detects glycoproteins separated by gel electrophoresis [SDS-PAGE] or two-dimensional gel electrophoresis [2D SDS-PAGE] and transferred to membranes. |
| Category | Sugar binding proteins |
| Protocol Name | |
Authors
 |
Yong Ma, Bruce
Research Center for Glycobiotechnology, Ritsumeikan University
|
| KeyWords |
|
Reagents
 |
| ● |
Purified glycoprotein or crude glycoprotein samples from cells etc. |
| ● |
All chemicals for gel electrophoresis |
| ● |
The transfer buffer for protein blotting |
| ● |
The nitrocellulose membranes or PVDF membranes (Bio-Rad Laboratories, Hercules, CA) |
| ● |
The filter papers (Whatman International Ltd., Kent, UK or Bio-Rad Laboratories) |
| ● |
Washing buffer: 0.5% (w/v) Tween-20 in Tris-buffered saline (TBST) or phosphate-buffered saline (PBST) |
| ● |
Blocking solution: 3% (w/v) bovine serum albumin (BSA) or 3% (w/v) skim milk in TTBS or TPBS |
| ● |
Biotinylated lectins and HRP-labeled extravidin or AP-labeled extravidin |
| ● |
Lectins, anti-lectin primary antibodies and HRP-labeled 2nd antibodies |
| ● |
Chromogenic or luminescent visualization regent for detecting tagged lectin or antibody |
| ● |
Two different detection methods: the chemiluminescent detection of HRP activity using the luminol reagent (PIERCE Kit) and the conventional colorimetric reaction of AP revealed by nitroblue tetrazolium/bromochloro-indolyl phosphate (NBT/BCIP) |
|
Instruments
 |
| ● |
Additional reagents and equipments for SDS-PAGE, immunoblotting and immunodetection |
| ● |
Image analyzer for chemiluminescence (e.g. LAS-4000, Fujifilm, Tokyo, Japan) |
|
| Methods |
|
1. |
SDS-PAGE or 2D SDS-PAGE electrophoresis |
| 1) |
Prepare the glycoprotein samples. |
Comment 0
|

|
| 2) |
Run on 5–20% SDS-PAGE or 2D SDS-PAGE gel until dye front is 0.5 cm from the bottom of the gel. |
Comment 0
|

|
|
|
|
2. |
Protein-blotting procedures |
| 1) |
Prepare transfer buffer while gel is running and degas for at least 1 h before transfer. |
Comment 0
|

|
| 2) |
Remove the gel plates carefully, and then transfer the gel. |
Comment 0
|

|
| 3) |
Wet the transfer nitrocellulose membrane thoroughly in a small amount of methanol. |
Comment 1
|

|
| 4) |
Wet the filter papers and the transfer membrane thoroughly in transfer buffer. |
Comment 0
|

|
| 5) |
Place transfer membrane on the gel, and make sure there are no bubbles. |
Comment 0
|

|
| 6) |
Place the 5 wetted pieces of filter papers on the membrane, and make sure there are no bubbles. |
Comment 0
|

|
| 8) |
Connect the lead to the power source, and transfer 1 h. |
Comment 0
|
|
|
|
3. |
Lectin blotting procedures |
| 1) |
After transfer of proteins from the gel to membrane, the membrane is removed and rinsed briefly in TBS or water. |
Comment 0
|

|
| 2) |
The membrane is then treated for 1 h at room temperature and under gentle agitation with 15 mL blocking solution (3% (w/v) BSA or 3% (w/v) skim milk in TBST). |
Comment 1
|

|
| 3) |
The blocking buffer is removed and the membrane is then incubated for 1–2 h in biotinylated lectin, at a concentration of 1 μg/mL in the blocking solution, under agitation, in a dish and at room temperature. 15 mL of the lectin solution are used for the incubation of a membrane of 9 × 9 cm. |
Comment 1
|

|
| 4) |
Blots are washed at least 4 times, 5 min each, with TBST. |
Comment 0
|

|
| 5) |
Extravidin-HRP or Extravidin-AP (at certain dilution) in the blocking solution is added for 1 h at room temperature under agitation. |
Comment 0
|

|
| 6) |
Blots are washed at least 4 times, 5 min each, with TBST and then rinsed twice with TBS. |
Comment 0
|

|
| 7) |
The chemiluminescence detection of peroxidase activity is performed with a SuperSignal West Pico Chemiluminescent kit according to the manufacturer’s instructions (PIERCE). The blots are incubated for 1 min at room temperature without agitation. |
Comment 0
|

|
| 8) |
The excess chemiluminescent solution is drained off by holding the blots vertically. The blots are then wrapped in plastic sheet (Saran Wrap), without introducing air bubbles. |
Comment 0
|

|
| 9) |
The luminescence is detected with an automatic developer or manually by electronic imaging systems. |
Comment 0
|

|
| 10) |
The exposure time of the blots depends on the amount of target proteins on the blots. |
Comment 1
|
|
|
| Discussion | Depending on the carbohydrate specificity of the lectin, it may be necessary to optimize working dilution. Notably, when the calcium dependent lectins such as C-type lectin are used, PBS must be replaced by other buffer system: TBS or Hepes buffer. Calcium phosphate precipitation occures.
When the lectin blotting method is combined with the high resolution and reproducibility of 2D SDS-PAGE and with the sensitivity of enhanced chemiluminescence, it is possible to identify rapidly the glycoproteins of interest by comparison with a reference 2D SDS-PAGE protein map, and to obtain reliable and reproducible results.
In most cases, lectins bind more strongly to oligosaccharides (di-, tri-, and tetrasaccharides) than to monosaccharides. Despite these limitations, lectin probes do provide some information as to the nature and composition of oligosaccharide substituents on glycoproteins. Their use together with blotting technique provides a convenient method of screening complex protein samples for abnormalities in the glycosylation of the component proteins. Lectin blotting requires low amounts of proteins, is easy to perform, and therefore is particularly indicated to be used in analyzing biological samples.
Many lectins recognize terminal nonreducing saccharides, whereas others also recognize internal sugar sequences. Moreover, lectins within each group may differ markedly in their affinity for the monosaccharides or their derivatives. They do not have an absolute specificity and therefore can bind with different affinities to a number of similar carbohydrate groups. Because lectin binding can also be affected by structural changes unrelated to the primary binding site, the results obtained with lectin-based methods must be interpreted with caution. |
| Figure & Legends |
Figure & Legends 
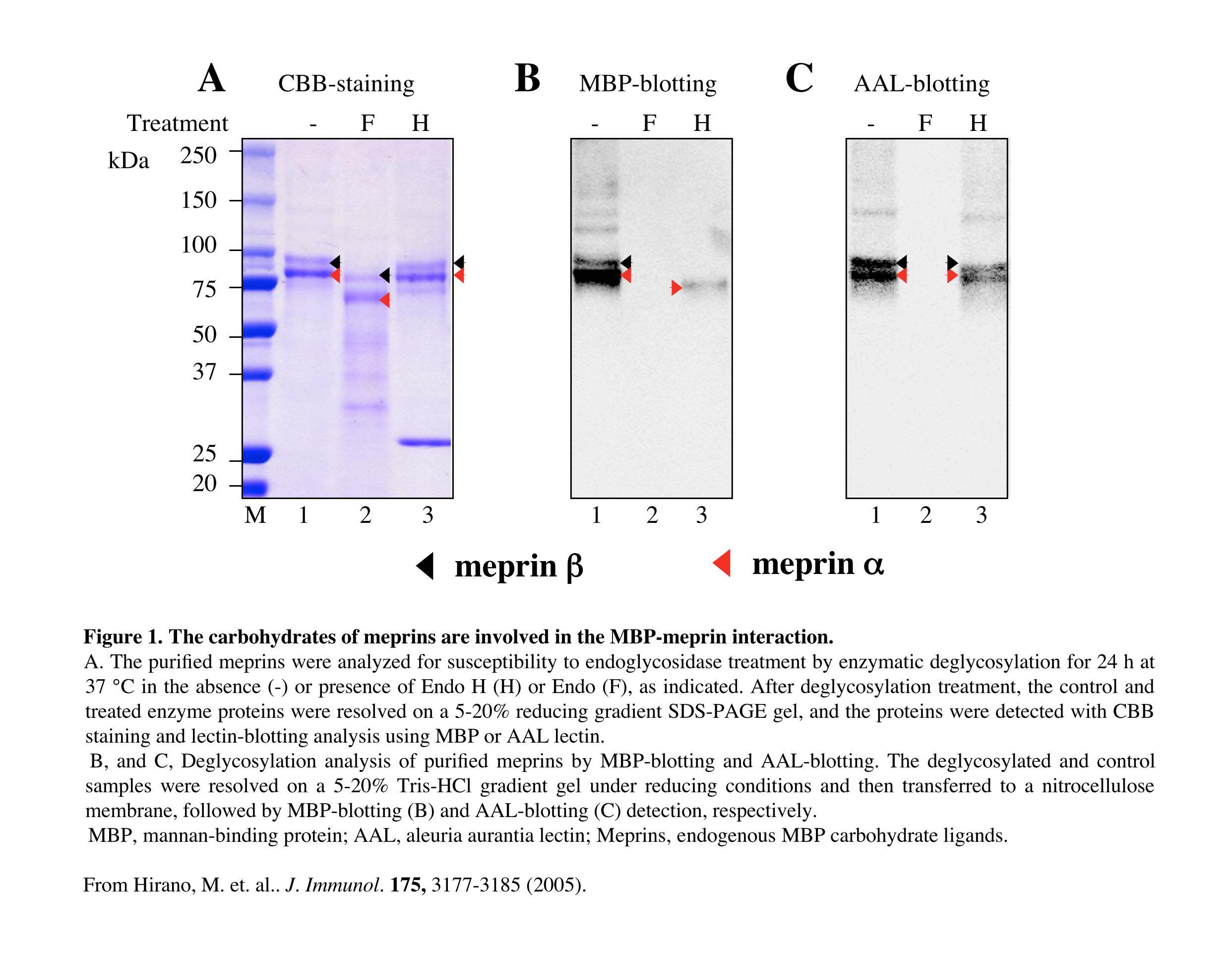
Fig. 1. Lectin blotting and deglycosylation analyses of the purified MBP ligands
Meprins, as novel endogenous MBP (mannan-binding protein) ligands, have been purified and identified through affinity chromatography and mass spectrometry. In order to characterize the oligosaccharides carried by meprins, and to investigate the interaction between MBP and the oligosaccharides of meprins, the oligosaccharides were removed enzymatically from meprins, and then the reactivity of the deglycosylated meprins toward MBP was examined by MBP-blotting and AAL-blotting analyses.
This figure was originally published in J Immunol. Hirano M, Ma BY. et al. "Mannan-binding protein blocks the activation of metalloproteases meprin alpha and beta" 2005, 175(5):3177–85. © The American Association of Immunologists, Inc.
|
| Copyrights |
Copyright 2005. The American Association of Immunologists, Inc. for Fig.1 in Figure & Legends
Copyright 2010. Ritsumeikan University, JCGGDB & AIST. for the rest of the contents |
| Date of registration:2015-02-03 13:37:16 |
- Hirano, M., Ma, B.Y., Kawasaki, N., Okimura, K., Baba, M., Nakagawa, T., Miwa, K., Kawasaki, N., Oka, S., and Kawasaki, T. (2005) Mannan-binding protein blocks the activation of metalloproteases meprin α and β. J. Immunol. 175, 3177–3185 [PMID : 16116208]
- Lis. H., and Sharon, N. (1986) Lectins as molecules and as tools. Annu. Rev. Biochem. 55, 35–67 [PMID : 3527046]
|
|
For those who wish to reuse the work, please contact JCGGDB management office (jcggdb-ml@aist.go.jp).
|
|