Mucins are highly glycosylated glycoproteins produced by epithelial cells and displayed/secreted to the luminal surfaces of these cells. Mucin gene products, the core polypeptides, are characterized by their tandem repeat domain rich in serine and threonine residues. In humans, 21 mucin genes were identified. They spread on different chromosomes except that the chromosome 11 has a small cluster of secreted mucins. Because of their epithelial origin, unique and mostly limited organ distribution, and their biochemical stability in body fluid, mucins are used as tissue and serum markers of carcinomas. Many mucin-specific monoclonal antibodies were generated in searches for carcinoma-specific epitopes.
The most extensively studied mucin is mucin-1(MUC1) and many anti-MUC1 monoclonal antibodies have been established. The majority of these antibodies recognize the peptide sequence corresponding to the tandem repeat portion and the affinity was enhanced or reduced by the glycosylation depending on the antibody and on the structural features of the carbohydrate chains. The enhancement is probably due to the epitope formed by a combination of peptides and the glycans and the reduction was due to concealment of the peptide epitope by glycans. There are MUC1-specific antibodies reactive regardless of the glycoforms, some of which are specific for sequences of the cytoplasmic tail. As examples, protocols for immunohistochemical localization of MUC1 in pancreas from human MUC1 transgenic mice are shown.
Availability of antibodies specific for other mucins is more limited than those specific for MUC1. Table 1 shows a list of these antibody currently published. We recently cloned mucin-21 (MUC21) (Itoh Y. et al. 2008). During our studies on the discovery and characterization of MUC21, polyclonal antibodies specific for the cytoplasmic tail of human MUC21 was prepared and immunohistochemistry was conducted. |
| Category | Sugar binding proteins |
| Protocol Name | Immunohistochemistry of mucin core proteins |
Authors
 |
Tian, Yuan
Laboratory of Cancer Biology and Molecular Immunology, Graduate School of Pharmaceutical Sciences, The University of Tokyo
Irimura, Tatsuro
*
Laboratory of Cancer Biology and Molecular Immunology, Graduate School of Pharmaceutical Sciences, The University of Tokyo
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Preparation of polyclonal antisera specific for the cytoplasmic tail of human MUC21
- A synthetic oligopeptide (C)RPVSSIAMEMSGRNSGP corresponding to the 17 residues at the carboxyl terminal of MUC21 is prepared by the use of peptide synthesizer (Applied Biosystems/Life Technologies, Carlsbad, CA) and purified by high performance liquid chromatography equipped with a C18 reverse-phase column (Nacalai Tesque Inc., Kyoto, Japan).
- The size of the oligopeptide is assessed by MALDI-TOF mass-spectroscopy with Voyager Elite.
- The peptide (1 mg) is conjugated to keyhole limpet hemocyanin (20 mg: Sigma-Aldrich, St. Louis, MO) with m-maleimidobenzoyl-N-hydroxysuccinimide ester through the attached cysteine residue added at the amino terminus.
- Japanese white rabbits are immunized twice with the conjugate with a two-week interval.
- The sera are obtained when the antibody titer is 2.4 × 104 (to reach a half maximum binding to the peptide) in ELISA two weeks after the second immunization. This antibody is named as anti-MUC21CT (Itoh Y. et al. 2008).
|
| ● |
Preparation of antibodies specific for human MUC1
- Preparations of antibodies specific for MUC1 are described elsewhere (Yamamoto M. et al. 1996; Higuchi T. et al. 2000)
|
|
| Methods |
|
1. |
Immunohistological staining of malignant and non-malignant tissue sections by the use of polyclonal antiserum specific for the cytoplasmic tail of MUC21 |
| 1) |
Tissues are preserved in 10% buffered formalin and embedded in paraffin and are serially sectioned at 4 μm thickness, mounted on silane-coated slides, and deparaffinized. |
Comment 0
|

|
| 2) |
The slides with sections are immersed for 30 min in 1% hydrogen peroxide in PBS to inactivate endogenous peroxidase. |
Comment 0
|

|
| 3) |
After washing 3 times with PBS for 5 min, the sections are incubated with 2% normal goat serum-3% bovine serum albumin/PBS (2% NGS-3% BSA/PBS) containing avidin (Vector Laboratories, Inc., Burlingame, CA) to block nonspecific antibody binding for 30 min at room temperature in a humidified chamber, then reacted with rabbit polyclonal antibody specific for cytoplasmic tail of human MUC21 (anti-MUC21CT: 1.4 mg/mL) at 1:1000 dilution in PBS containing 2% NGS-3% BSA with biotin (Vector Laboratories) overnight at 4°C. |
Comment 0
|

|
| 4) |
After washing with PBS, biotin labeled goat anti-rabbit IgG (0.75 mg/mL) was added at 1: 200 dilution in 2% NGS-3% BSA/PBS as the second antibody. |
Comment 0
|

|
| 5) |
The antibody binding was visualized by the use of peroxidase-conjugated streptavidin (1.25 mg/mL) at 1: 100 dilution in 2% NGS-3% BSA/PBS following incubation with diethylaminobenzidine (Vector Laboratories). |
Comment 0
|
|
|
|
2. |
Immunohistological staining of tissue sections by the use of antibodies specific for different domains of MUC1 |
| 1) |
Two antibodies, mAb MY.1E12 (anti-MUC1 with sialyl-T antigen) (Yamamoto M. et al. 1996; Takeuchi H. et al. 2002) and hen polyclonal IgY antibody specific for synthetic peptide corresponding to the cytoplasmic portion (carboxyl terminal) of MUC1, SSLSYTNPAVAATSANL (Higuchi T. et al. 2000), are used. |
Comment 0
|

|
| 2) |
OCT-embedded frozen tissues from normal pancreas of a MUC1-transgenic mouse are serially sectioned at 5 μm, mounted on poly-L-lysine-coated slides, and fixed in cold ethanol for 30 sec. |
Comment 0
|

|
| 3) |
Slides are washed 3 times for 5 min each in PBS, and immersed in 0.3% hydrogen peroxide in PBS 3 times for 5 min to inactivate endogenous peroxidase. |
Comment 0
|

|
| 4) |
The sections are incubated with avidin diluted in blocking solution (2% NGS, 2% normal mouse serum [NMS], 3% BSA in PBS) at room temperature for 15 min, washed 3 times for 5 min each in PBS, then incubated with biotin in the blocking solution for 15 min, and washed 3 times for 5 min each in PBS. |
Comment 0
|

|
| 5) |
The sections are incubated with 5 μg/mL of biotin conjugated mAb MY.1E12, or biotin conjugated hen polyclonal IgY antibody as primary antibodies at 4°C overnight. |
Comment 0
|

|
| 6) |
Biotin conjugated-IgG2a (BioLegend, San Diego, CA) (5 μg/mL) or biotin-conjugated IgY (Santa Cruz Biotechnology, Santa Cruz, CA) (5 μg/mL) are used as isotype controls. |
Comment 0
|

|
| 7) |
The sections are washed with PBS, incubated with HRP-avidin (Vector Laboratories) diluted by of 1:200 in blocking solution at room temperature for 30 min, washed, incubated with diaminobenzidine staining kit (Vector Laboratories) until a sufficient color developed, counterstained in Mayer’s Hematoxylin, and mounted in Entellan Neu (Merck Millipore, Billerica, MA). |
Comment 0
|
|
|
| Figure & Legends |
Figure & Legends
| Mucin |
Antibody |
Epitope |
Source / Reference |
| MUC2 |
3A2 |
VNTR domain |
Jass JR, University of Queensland, Australia. (winterford et al., 1999) |
| Ccp58 |
VNTR domain |
Novocastra UK (Glickman et al., 2003) |
| PH1417 |
C-terminus (aa 4995–5013) |
G. Hansson, Go¨teburg University, Sweden (Axelsson et al., 1998) |
| PH1491 |
N-terminus (aa 1167–1180) |
G. Hansson, Go¨teburg University, Sweden (Asker et al., 1998) |
| H-300 (polyclonal) |
amino acids 4880-5179 mapping at the C-terminus |
Santa Cruz Biotechnology, USA |
| MUC3 |
M3.1 (ab24068) |
TR |
Abcam, UK |
| N-20 (polyclonal) |
peptide mapping within an internal region of Mucin 3A |
Santa Cruz Biotechnology, USA |
| SPM200 |
TR |
Santa Cruz Biotechnology, USA |
| 3H2744 |
TR |
Santa Cruz Biotechnology, USA |
| MUC4 |
8G7 |
TR |
Batra SK, University of Nebraska Medical Center, Nebraska (Moniaux et al., 2004) |
| M4.275 |
VNTR domain |
Jass JR, University of Queensland, Australia. (winterford et al., 1999) |
| 1G8 |
Ectodomain of ASGP-2 |
Zymed, San Francisco, USA (Zhang et al., 2005) |
| MUC5AC |
CLH2 |
TR |
U. Mandel, University of Copenhagen, Denmark (Reis et al., 1997); Vector Labs, Burlingame, CA |
| 45M1 |
core polypeptide |
Novocastra UK (Glickman et al., 2003) |
| 1-13M1 |
N-terminus: cys-2, cys-4 domains |
NeoMarkers, Fremont, USA (Nollet et al., 2004) |
| 791 |
D3 domin |
I.K. Gipson (Argueso et al., 2002) |
| MUC5B |
799 |
D4 domain |
I.K. Gipson (Gipson et al., 2001) |
| Eu-MUC5Ba |
Cysteine-rich domain |
D.M. Swallow, University College, London, UK (Rousseau et al., 2003) |
| H-300 (polyclonal) |
amino acids 1201-1500 mapping near the C-terminus |
Santa Cruz Biotechnology, USA |
| 4H310 |
a peptide mapping at the
N-terminus
|
Santa Cruz Biotechnology, USA |
| MUC6 |
CLH5 |
core polypeptide |
Novocastra, UK (Glickman et al., 2003) |
| 0.N.459 |
TR |
Santa Cruz biotechnology, USA |
| MUC7 |
PANH3 |
N-terminus (aa 72-92) |
U. Mandel, University of Copenhagen, Denmark (Nielsen et al., 1996) |
| Eu-MUC7a |
Histatin-like domain |
D.M. Swallow, University College, London, UK (Rousseau et al., 2003) |
| H-150( polyclonal) |
amino acids 1-75 mapping at the N-terminus |
Santa Cruz Biotechnology, USA |
| MUC12 |
ab2298 (polyclonal) |
amino acids 541-555 |
Abcam UK (Williams et al., 1999) |
| ab3162 (polyclonal) |
SSISGEPTSLYSQAE |
Abcam UK (Williams et al., 1999) |
| MUC13 |
ppz0020 |
EGF2 domain |
Aburatani H, University of Tokyo, Japan
(Shimamura et al., 2005)
|
| ppz0025 |
N-terminus |
Aburatani H, University of Tokyo, Japan
(Shimamura et al., 2005)
|
| ST0751(polyclonal) |
C-terminus |
Aburatani H, University of Tokyo, Japan (Shimamura et al., 2005) |
| ab65109 (polyclonal) |
internal sequence |
Abcam, UK |
| ab72050 (polyclonal) |
residues 480-530 |
Abcam, UK |
| H-300 (polyclonal) |
amino acids 19-318 mapping within an N-terminal extracellular domain
|
Santa Cruz Biotechnology, USA |
| MUC15 |
HPA026110 (polyclonal) |
Tag (PrEST) antigen sequence |
Sigma-Aldrich |
| C-15(polyclonal) |
C-terminal cytoplasmic domain |
Santa Cruz Biotechnology, USA |
| H-210 (polyclonal) |
amino acids 24-233 mapping within an N-terminal extracellular domain |
Santa Cruz Biotechnology, USA |
| MUC16 |
OC125 |
TR |
Dako Corporation, Carpenteria, USA (Bast et al., 1981) |
| M11 |
TR |
NeoMarkers, Fremont, USA (Nustad et al., 1996) |
| H-150 (polyclonal) |
amino acids 6419-6568 mapping near the C-terminus |
Santa Cruz Biotechnology, USA |
| MUC18 |
ab28360 (polyclonal) |
amino acids 433-647 |
Abcam, UK (Frittsche et al., 2008) |
| ab75862(polyclonal) |
amino acid residues surrounding Y641 of human CD146 |
Abcam, UK |
| EPR3208(ab75769) |
intracellular sequence |
Abcam, UK |
| N1238(ab49492) |
external domain |
Abcam, UK |
| MUC20 |
ab73043 (polyclonal) |
Full length protein, corresponding to amino acids 1-538 |
Abcam, UK |
| 13380-1-AP (polyclonal) |
255-555aa |
Proteintech Group, USA |
Table 1 List of antibodies specific for core polypeptides of human mucins

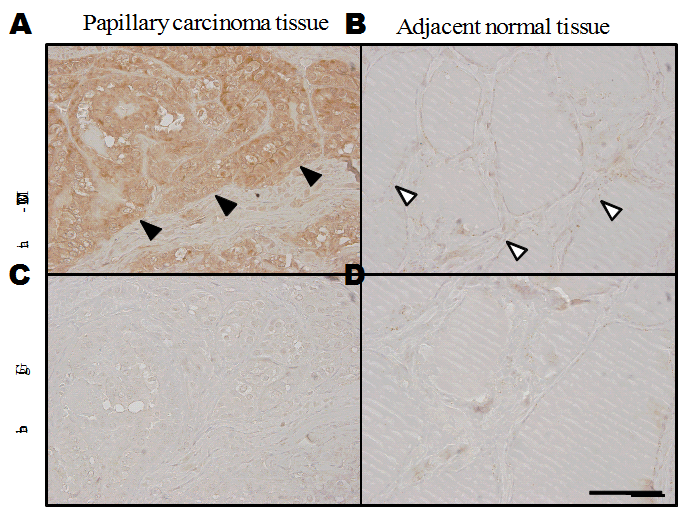
Fig. 1. Immunohistochemical staining of human tissue with anti-MUC21CT
(A) Papillary carcinoma of thyroid showing strong antibody reactivity to carcinoma cells. The site of antibody binding appeared to be the cytoplasm. (B) The antibody did not bind normal thyroid follicles adjacent to the papillary carcinoma. (C) A section of papillary carcinoma of thyroid representing the same area as (A) was reacted with rabbit IgG. (D) A section of normal thyroid follicles reacted with rabbit IgG. The bar indicates 100 μm. We thank Dr. Toshihisa Ogawa of Department of Breast and Endocrine Surgery, Faculty of Medicine, the University of Tokyo for providing the thyroid sections.

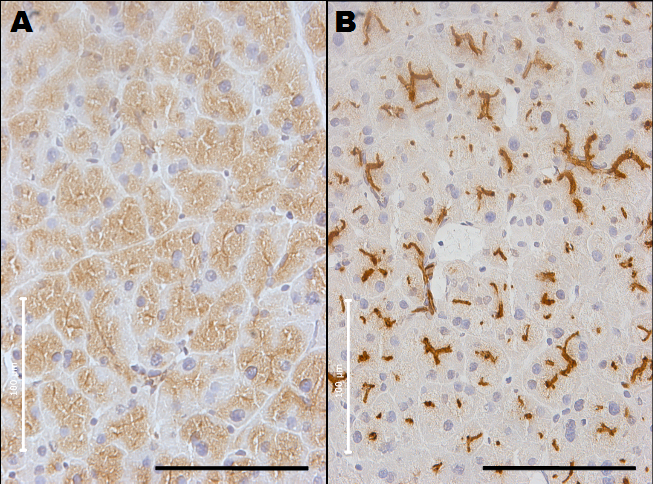
Fig. 2. Immunohistochemical staining of normal pancreas of MUC1 transgenic mouse with anti-MUC1 antibodies
(A) A section of pancreatic duct stained with purified polyclonal hen antibody specific for carboxyl terminal of human MUC1. (B) A section of pancreatic duct was reacted with mAb MY.1E12 specific for MUC1 with sialyl-T epitope. The bar indicates 100 μm. Binding sites of antibody specific for C-terminal portion of human MUC1exhibied cytoplasmic distribution whereas binding sites of the antibody specific for human MUC1 with sialyl-T epitope distributed on the luminal surfaces. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-08-26 16:02:33 |
- Yamamoto, M., Bhavanandan, V.P., Nakamori, S., and Irimura, T. (1996) A novel monoclonal antibody specific for sialylated MUC1 mucin. Jpn J Cancer Res. 87, 488–496 [PMID : 8641986]
- Takeuchi, H., Kato, K., Denda-Nagai, K., Hanisch, F.G., Clausen, H., and Irimura T. (2002) The epitope recognized by the unique anti-MUC1 monoclonal antibody MY.1E12 involves sialylα2-3galactosylβl-3N-acetylgalactosaminide linked to a distinct threonine residue in the MUC1 tandem repeat. J Immunol Methods. 270, 199–209 [PMID : 12379325]
- Higuchi, T., Xin, P., Buckley, M.S., Erickson, D.R., Bhavanandan, V.P. (2000) Characterization of the rabbit homolog of human MUC1 glycoprotein isolated from bladder by affinity chromatography on immobilized jacalin. Glycobiology 10, 659–667 [PMID : 10910971]
- Itoh, Y., Kamata-Sakurai, M., Denda-Nagai, K., Nagai, S., Tsuiji, M., Ishii-Schrade, K., Okada, K., Goto, A., Fukayama, M., and Irimura, T. (2008) Identification and expression of human epiglycanin/MUC21: a novel transmembrane mucin. Glycobiology 18, 74–78 [PMID : 17977904]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Tian, Yuan,
Irimura, Tatsuro,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.23,4,2024 .
How to Cite this Work in Website:
Tian, Yuan,
Irimura, Tatsuro,
(2014).
Immunohistochemistry of mucin core proteins.
Retrieved 23,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t53.
html source
Tian, Yuan,
Irimura, Tatsuro,
(2014).
<b>Immunohistochemistry of mucin core proteins</b>.
Retrieved 4 23,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t53" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t53</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|