In-line flow glycan-releasing system, “AutoGlycoCutter (AGC)”, enables rapid release of reactive reducing O-linked glycans from the core protein. The released O-glycans are successfully labeled with highly sensitive fluorescent reagents such as 2-aminobenzoic acid, and can be analyzed by HPLC and CE with high sensitivity. The method described here are applicable to biological samples such as cultured cells, tissues and serum samples. |
| Category | O-Glycans |
| Protocol Name | Rapid and sensitive analysis of O-glycans using an in-line flow glycan-releasing system |
Authors
 |
Kinoshita, Mitsuhiro
Faculty of Pharmaceutical Sciences, Kinki University
Kakehi, Kazuaki
*
Faculty of Pharmaceutical Sciences, Kinki University
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
Pronase (Streptomyces griseus) (Calbiochem, San Diego, CA) |
| ● |
2-Aminobenzoic acid (2-AA) (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan) |
| ● |
Sodium borohydride (NaBH4) (Sigma-Aldrich, St. Louis, MO) |
| ● |
Sodium cyanoborohydride (NaCNBH3) (Sigma-Aldrich) |
| ● |
2,5-Dihydroxybenzoic acid (DHB) (Sigma-Aldrich) |
|
Instruments
 |
| ● |
AutoGlycoCutter (AGC) (Shimadzu Corporation, Kyoto, Japan) |
| ● |
Voyager DE-Pro mass spectrometer (Applied Biosystems-Life Technologies, Carlsbad, CA) |
| ● |
Speed Vac Concentrator (Thermo Fisher Scientific Inc., Waltham, MA) |
| ● |
Ultrafree-MC (MWCO 5000, Merck Millipore, Billerica, MA) |
| ● |
Sephadex LH-20 column (1 × 30 cm) (GE Healthcare, Little Chalfont, UK) |
|
| Methods |
|
1. |
Preparation of Glycopeptide Pool from biological samples.
In the releasing reaction of O-linked glycans, biological samples such as cultured cells, tissues or serum, are digested with proteases prior to the releasing reaction. For the releasing reaction of O-linked glycans of purified soluble glycoproteins, the following procedures can be omitted. |
| 1) |
Cultured cells (~1.0 × 107 cells) or a tissue sample (~50 mg as wet weight) are suspended in 5 mM Tris-HCl buffer (pH 8.0, 500 μL), and mixed with an equal volume of 2% Triton-X 100 in the same buffer in an ice bath. |
Comment 0
|

|
| 2) |
After homogenizing the cells for 7 min with a glass homogenizer, the mixture is centrifuged at 8,000 × g for 30 min. |
Comment 0
|

|
| 3) |
The supernatant layer is collected and boiled for 7 min at 100°C, and evaporated to dryness by a centrifugal evaporator. |
Comment 0
|

|
| 4) |
The lyophilized material is suspended in water (200 μL), and ethanol (800 μL) is added to the mixture to 80% concentration. |
Comment 0
|

|
| 5) |
The precipitate is collected by centrifugation and washed with ethanol (1 mL × 3) and then with acetone (1 mL × 2)*. |
Comment 1
|

|
| 6) |
The residue is dried in vacuo, and digested with pronase (50 μg) in 5 mM Tris-HCl (pH 8.0, 200 μL) at 37°C for 24 h. |
Comment 0
|

|
| 7) |
The reaction mixture is boiled for 10 min, and the supernatant is collected after centrifugation. |
Comment 0
|

|
| 8) |
An aqueous solution of 2 M NaBH4 (500 μL) is added to the supernatant, and kept at room temperature for 30 min*. |
Comment 1
|

|
| 9) |
Glacial acetic acid is carefully added to the mixture to decompose excess NaBH4, and the mixture is passed through an ultrafiltration membrane (MW 5000 cutoff, Ultrafree-MC) at 10,000 × g. |
Comment 0
|

|
| 10) |
The mixture of glycopeptides on the membrane is dissolved in water (250 μL), and used for releasing reaction of O-glycans. |
Comment 0
|
|
|
|
2. |
Releasing of reducing O-linked glycans using AGC |
| 1) |
The reaction coil (0.25 mM i.d., 10 m length) is filled with 0.5 M LiOH, and the cation exchange cartridge is equilibrated with distilled water, and the temperature of the reaction coil in the reactor is set at 60°C* (Fig. 1). |
Comment 1
|

|
| 2) |
An aqueous solution of the glycoproteins* or glycopeptides is injected into the AGC, in which 0.5 M LiOH is used as eluent at 1.0 mL/min (0.7 min for the releasing reaction). The releasing reaction occurs during passing through the reaction coil. |
Comment 1
|

|
| 3) |
The reaction mixture from the reaction coil is immediately introduced to the cartridge packed with cation-exchange resin (ca. 1 mL volume) is desalted. |
Comment 0
|

|
| 4) |
The eluate from the cartridge is collected into the fraction collector by monitoring the UV absorbance at 230 nm. |
Comment 0
|

|
| 5) |
After the run, the cation exchange cartridge is regenerated with 0.25 M H2SO4 at a flow rate of 1.2 mL/min for 5 min, followed by washing it with water at the same flow rate for 5 min.* |
Comment 1
|

|
| 6) |
The collected sample solution (ca. 900 μL) containing the released O-glycans is evaporated to dryness by a centrifugal evaporator. |
Comment 0
|
|
|
|
3. |
Fluorescent labeling of reducing oligosaccharides with 2-AA |
| 1) |
The dried material obtained as described above are dissolved in 2-AA solution (100 μL), freshly prepared by dissolution of 2-AA* and sodium cyanoborohydride (15 mg each) in methanol (1 mL) containing 4% sodium acetate and 2% boric acid. |
Comment 1
|

|
| 2) |
The mixture is kept at 80°C for 1 h. |
Comment 0
|

|
| 3) |
After cooling, water (100 μL) is added, and the mixture is applied to a column of Sephadex LH-20 (1.0 cm i.d., 30 cm) previously equilibrated with 50% aqueous methanol. |
Comment 0
|

|
| 4) |
The earlier eluted fluorescent fractions are pooled and evaporated to dryness under reduced pressure. |
Comment 0
|

|
| 5) |
The residue is dissolved in water (100 μL), and a portion (10 μL) is used for the analysis by normal-phase high-performance liquid chromatography (NP–HPLC), MALDI-TOF MS, and capillary electrophoresis (CE). |
Comment 0
|
|
|
| Notes | By this procedure, we can analyze O-linked glycans from the glycopeptides obtained from at least 2.0 × 106 cells.
Because we can obtain the O-linked glycans as a reactive reducing form by using an in-line-flow system, the glycans are labeled with fluorescent tags for high sensitive and high resolution analysis. A typical example of the analysis of O-linked glycans from cultured cells is shown in Fig. 2. And the results are summarized in Table 1. All the cells other than HL-60 show relatively simple chromatograms. The peaks in the chromatogram are collected and analyzed by MALDI-QIT-TOF MS. All leukemia cells commonly contain sialyl-T and disialyl-T as the major glycans. Jurkat cells contain some other glycans such as sialyl-Tn (J2) and core2-type disialyl hexasaccharide (J4). HL-60 cells also contain sialyl-T (H4) and core2-type disialyl hexasaccharide (H13). O-Glycans of core2-type glycans having some lactosamine units (Gal-GlcNAc) are also observed in HL-60 cells. Although most leukemia cells do not show the activity of core2 enzyme, HL-60 cells express significant activity of that enzyme. Profiles of O-linked glycans obtained by this procedure are well correlated with their biochemical characteristics.
This procedure can also be applicable to the release of the GAGs part of PGs. After digestion of aggrecan, which is the major PG in articular cartilage, with chondroitinase ABC, the oligosaccharides at the linkage region are successfully released from the core protein as shown in Fig. 3. Two major oligosaccharides are observed on the CE electropherogram. These peaks are not digested with chondro-4-sulfatase and alkaline phosphatase. Thus, we can conclude that peak 1 and 2 are hexasaccharides having a sulfate group at the C6 position of the GalNAc residue and nonsulfated hexasaccharide, respectively. The structures of hexasaccharides at the linkage region observed in aggrecan correspond to those reported previously.
The procedure described here is a useful and powerful tool for rapid release of O-linked glycans from glycoproteins and for high sensitive quantitative analysis of O-linked glycans. The releasing reaction of glycans is also highly reproducible by an automatic in-line-flow system. It is easy to carry out comparative quantitative analysis of O-linked glycans in biological samples such as cultured cells, tissues, or serum. |
| Figure & Legends |
Figure & Legends 
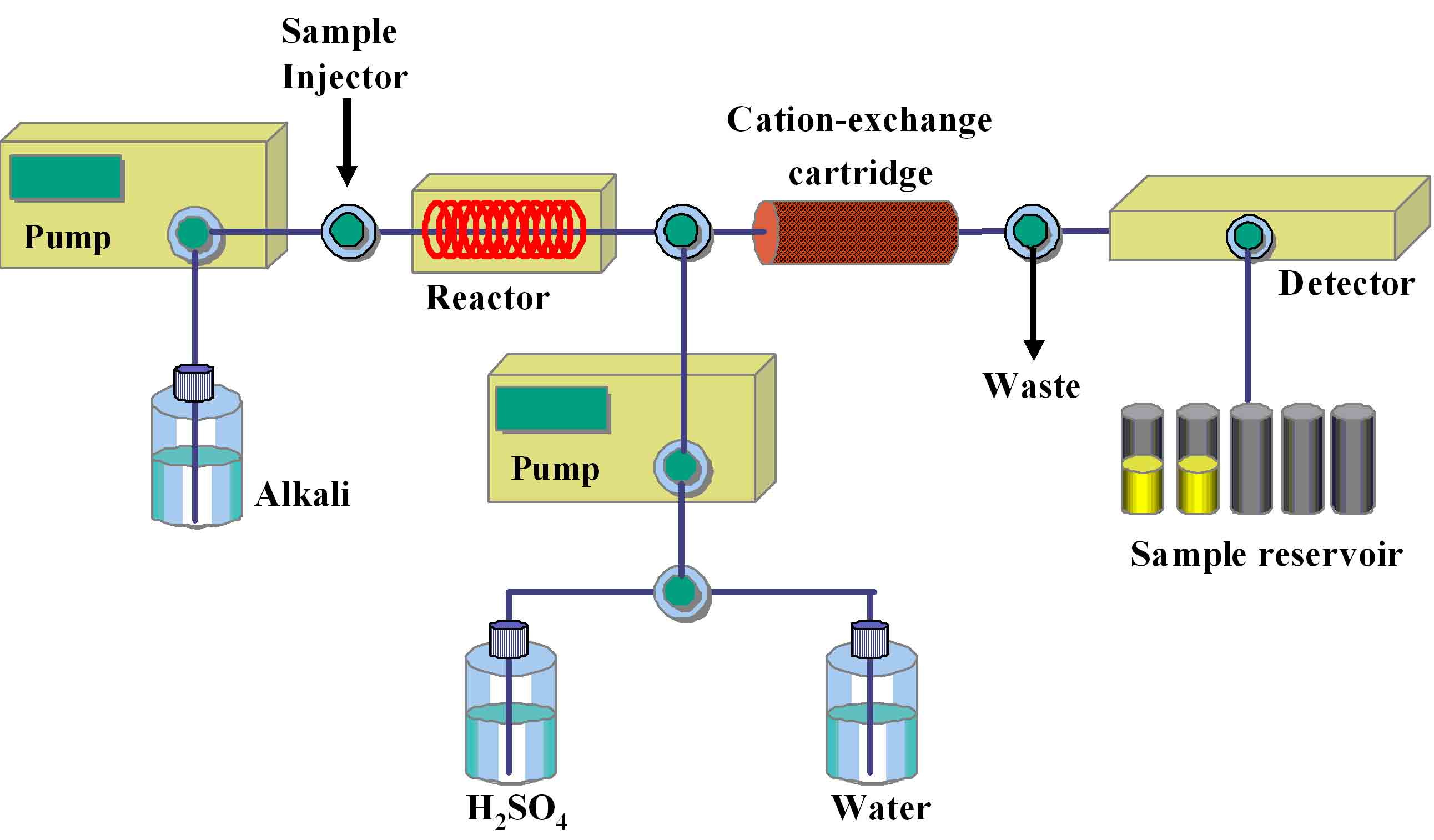
Fig. 1. AutoGlycoCutter (AGC) for the release of O-linked glycans from the core protein
AGC is an automatic apparatus composed of several units. A portion of the sample solution is introduced by an automatic injector into the flow of 0.5 M LiOH solution supplied by a pump at a flow rate of 0.5 mL/min. O-Linked glycans are released in the reaction coil thermostated at constant temperature. The solution containing the released oligosaccharides is immediately cooled and neutralized by passing through a cation-exchange cartridge to avoid degradation of the released oligosaccharides. The effluent from the cartridge is collected by a fraction collector. After the reaction, the cartridge is regenerated with 0.25 M H2SO4 at a flow rate of 1.2 mL/min followed by washing with water by an automatic valve changing device.

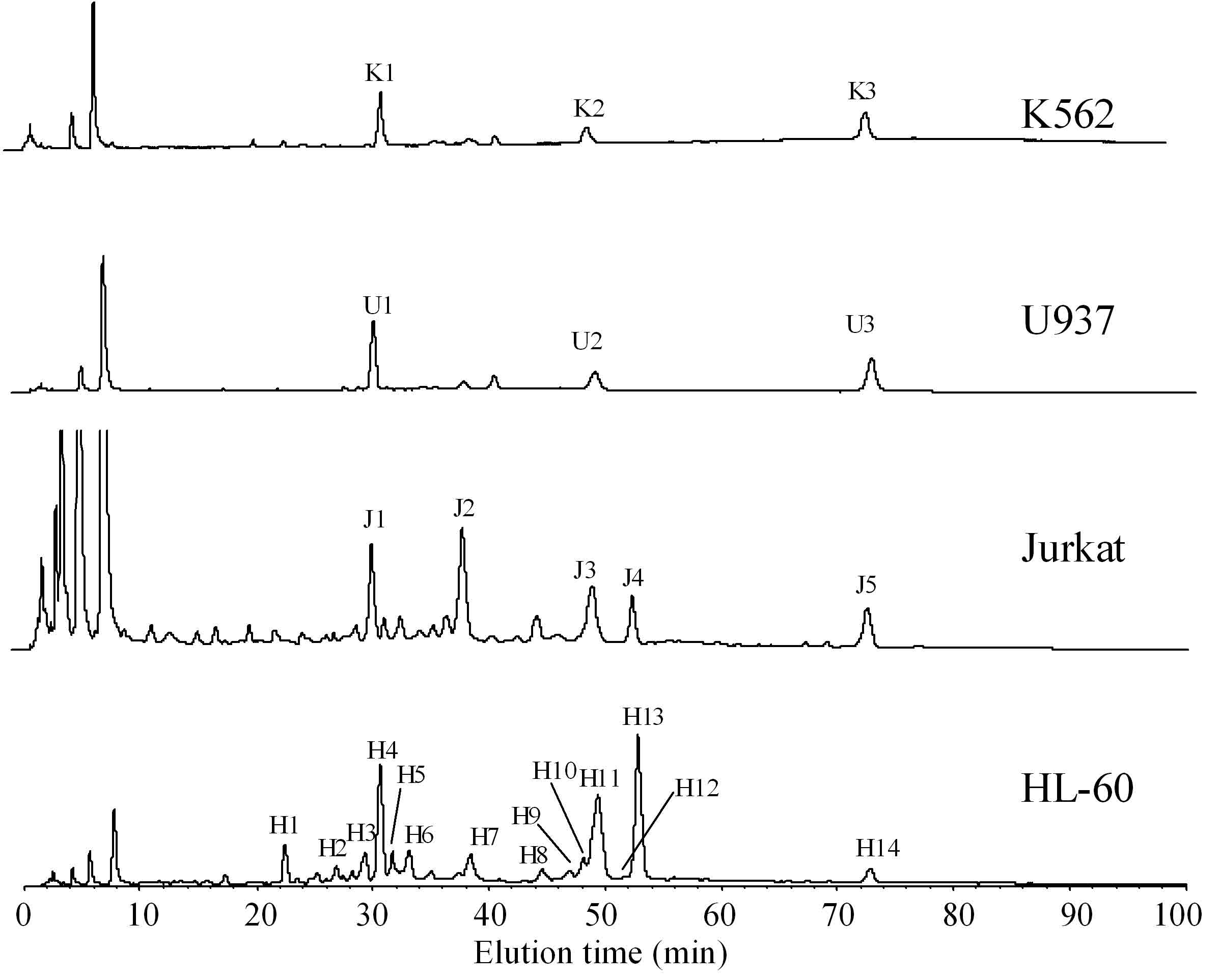
Fig. 2. NP-HPLC analysis of 2-AA labeled O-linked glycans derived from leukemia cells
O-Linked glycans derived from K562, U937, Jurkat, and HL-60 cells are released by AGC followed by labeling with 2-AA. Analytical conditions for HPLC: column, Asahi Shodex NH2P-50 4E (4.6 × 250 mm; Showa Denko K.K., Tokyo, Japan); eluent, solvent A, 2% acetic acid in acetonitrile; solvent B, 5% acetic acid in water containing 3% triethylamine; gradient condition, linear gradient (30–95% solvent B) from 2 to 82 min, maintained for 20 min. Fluorescence detection is performed at 425 nm irradiated with a 350-nm light. Identification of the observed peaks is performed with MALDI-TOF mass spectrometry. Oligosaccharides structures are summarized in Table 1.
This figure was originally published in "Comparative studies on the structural features of O-glycans between leukemia and epithelial cell lines" Yamada K, Kinoshita M. et al. [J Proteome Res. 8(2):521–37] 2009 Feb. DOI: 10.1021/pr800710f.

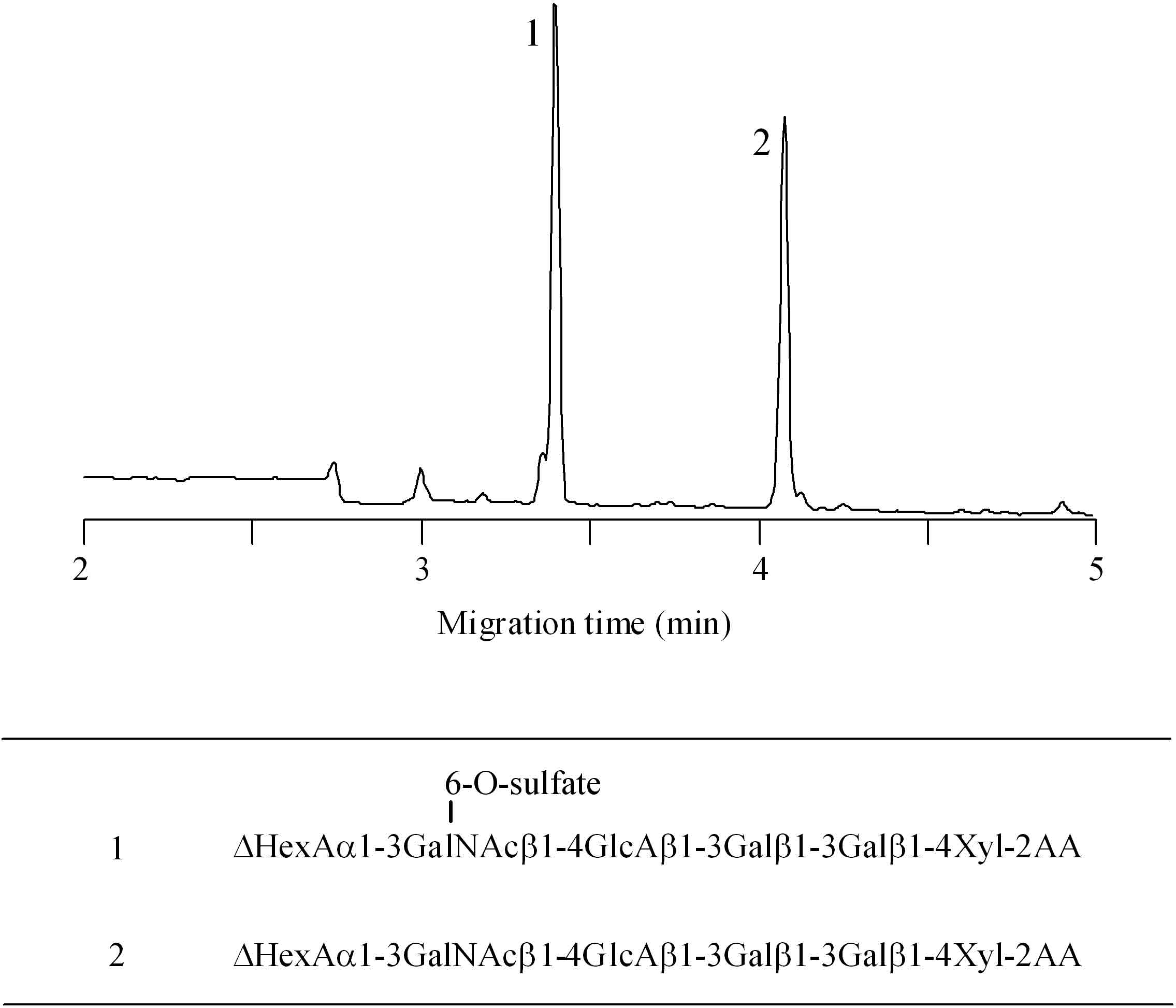
Fig. 3. CE analysis of 2-AA labeled linkage oligosaccharides derived from aggrecan
Analytical conditions for CE: column, DB-1 capillary (100 μm i.d. × 30 cm); electrolyte, 0.1 M Tris-borate (pH 8.0) containing 10% PEG70000; applied voltage, 20 kV; injection, pressure method (1.0 psi, 10 sec); detection, He-Cd laser-induced fluorescence (Ex. 325 nm, Em 405 nm).
Reprinted from Anal Biochem., 362(2), Matsuno YK, Kinoshita M, Kakehi K. et al., Development of an apparatus for rapid release of oligosaccharides at the glycosaminoglycan-protein linkage region in chondroitin sulfate-type proteoglycans, 245–57, 2007, with permission from Elsevier. doi:10.1016/j.ab.2006.12.027.

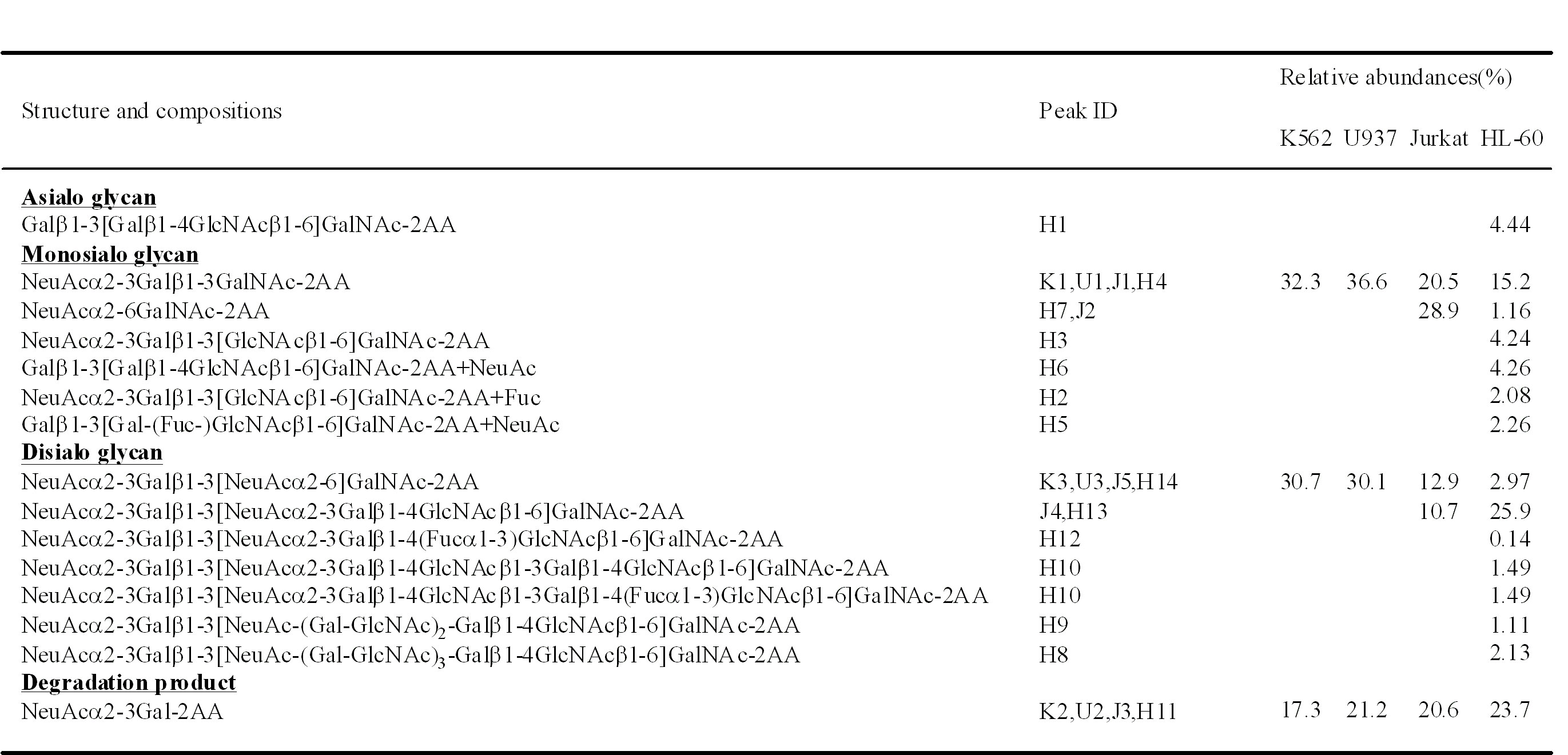
Table 1. O-Linked glycans in leukemia cells
This table was originally published in "Comparative studies on the structural features of O-glycans between leukemia and epithelial cell lines" Yamada K, Kinoshita M. et al. [J Proteome Res. 8(2):521–37] 2009 Feb. DOI: 10.1021/pr800710f.
|
| Copyrights |
Copyright 2007. Elsevier, for Fig.3 in Figure & Legends
Copyright 2010. Ritsumeikan University, JCGGDB & AIST. for the rest of the contents |
| Date of registration:2014-06-13 13:28:18 |
- Yamada, K., Watanabe, S., Kita, S., Kinoshita, M., Hayakawa, T., and Kakehi, K. (2010) Determination of Tn antigen released from cultured cancer cells by capillary electrophoresis. Anal. Biochem. 396, 161–163 [PMID : 19699708]
- Yamada, K., Kinoshita, M., Hayakawa, T., Nakaya, S., and Kakehi, K. (2009) Comparative studies on the structural features of O-glycans between leukemia and epithelial cell lines. J. Proteome Res. 8, 521–537 [PMID : 19154102]
- Yamada, K., Hyodo, S., Matsuno, Y.K., Kinoshita, M., Maruyama, S.Z., Osaka, Y.S., Casal, E., Lee, Y.C., and Kakehi, K. (2007) Rapid and sensitive analysis of mucin-type glycans using an in-line flow glycan-releasing apparatus. Anal. Biochem. 371, 52–61 [PMID : 17632070]
- Matsuno, Y.K., Yamada, K., Tanabe, A., Kinoshita, M., Maruyama, S.Z., Osaka, Y.S., Masuko, T., Kakehi, K. (2007) Development of an apparatus for rapid release of oligosaccharides at the glycosaminoglycan-protein linkage region in chondroitin sulfate-type proteoglycans. Anal. Biochem. 362, 245–257 [PMID : 17250796]
|
|
For those who wish to reuse the work, please contact JCGGDB management office (jcggdb-ml@aist.go.jp).
|
|