| Category | Glycosyltransferases & related proteins |
| Protocol Name | Enzyme assay of polypeptide N-acetylgalactosaminyltransferase, β1,3-glycosyltransferase, ang β1,4-glycosyltransferases. [3] β1,4-glycosyltransferases family |
Authors
 |
Sato, Takashi
Research Center for Medical Glycoscience, National Institute of Advanced Industrial Science and Technology (AIST)
Ito, Hiromi
Department of Biochemistry, Fukushima Medical University School of Medicine
Narimatsu, Hisashi
*
Research Center for Medical Glycoscience, National Instutute of Advanced Industrial Science and Technology (AIST)
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
Various acceptor substrates |
| ● |
Recombinant β1,4 galactosyltransferase enzymes |
| ● |
Recombinant β1,4 N-acetylgalactosaminyltransferase enzymes |
| ● |
Biotinylated Ricinus communis agglutinin I (RCA-I) |
| ● |
Biotinylated Wisteria floribunda lectin (WFA, WFL) |
|
Instruments
 |
|
| Methods |
|
1. |
β1,4-galactosyltransferase assay (see Note 1) |
| 1) |
Mix the following components in a small plastic tube.
20 mM HEPES buffer (pH 7.0)
10 mM MnCl2
2.5 μM UDP-Gal
Various accepter substrates
5 μL of enzyme-agarose gel suspension or free soluble enzyme |
Comment 0
|

|
| 2) |
Incubate the mixture at 37°C for various periods with agitation. |
Comment 0
|

|
| 3) |
Terminate the enzyme reaction by heating at 100°C for 3 min. |
Comment 0
|

|
| 4) |
Centrifuge the reaction mixture at 15,000 rpm for 5 min at 4°C and recover the supernatant. |
Comment 0
|

|
| 5) |
Apply the supernatant to an HPLC on the suitable columns to separate the substrate and product. Select the separation method of reaction product depending on accepter substrate you used. When glycoprotein is used as an accepter substrate, Ricinus Communis Agglutinin (RCA) blot is suitable for detection of reaction product. |
Comment 0
|
|
|
|
2. |
β1,4-N-acetylgalactosaminyltransferase assay (see Note 2) |
| 1) |
Mix the following components in a small plastic tube.
50 mM MES buffer (pH 6.5)
0.1% Triton X-100
10 mM MnCl2
100 μM UDP-GalNAc
Various accepter substrates
5 μL of enzyme-agarose gel suspension or free soluble enzyme |
Comment 0
|

|
| 2) |
Incubate the mixture at 37°C for various periods with agitation. |
Comment 0
|

|
| 3) |
Terminate the enzyme reaction by heating at 100°C for 3 min. |
Comment 0
|

|
| 4) |
Centrifuge the reaction mixture at 15,000 rpm for 5 min at 4°C and recover the supernatant. |
Comment 0
|

|
| 5) |
Apply the supernatant to an HPLC on the suitable columns to separate the substrate and product. Select the separation method of reaction product depending on accepter substrate you used. When glycoprotein is used as an accepter substrate, wisteria floribunda lectin blot is suitable for detection of reaction product. |
Comment 0
|
|
|
| Notes | 1) β1,4-Galactosyltransferase (β4Gal-T)
In mammals, seven β1,4-galactosyltransferases have been identified and all of them transfer galactose (Gal) from UDP-Gal as a donor substrate toward a variety of accepter substrates via β1-4 linkage. Preference of the acceptor substrates is different in each enzyme. β4Gal-T1 is the first mammalian glycosyltransferase that the gene was cloned in 1986 (1). This enzyme has dual glycosyltransferase activity to transfer Gal toward the acceptor substrate N-acetylglucosamine (GlcNAc) synthesizing N-acetyllactosamine (Galβ1,4GlcNAc), and toward the acceptor substrate glucose (Glc) synthesizing lactose (Galβ1,4Glc) in cooperation with lactalbumin. Similar to β4Gal-T1, β4Gal-T2, -T3, -T4 and -T5 synthesize Galβ1,4GlcNAc-, but in vitro analysis revealed that these enzymes exhibit different specificity of acceptor substrates each other (2). β4Gal-T4 is a keratan sulfate synthase which also uses GlcNAc-6-sulfate, a constituent of keratan sulfate, as a substrate (3). β4Gal-T5 and β4Gal-T6 use Glc-ceramide (Glc-Cer) as the acceptor substrate and shows lactosyl-Cer synthase activity (4-6). β4Gal-T7 uses xylose-serine (Xyl-Ser) in the proteoglycan core protein as the acceptor substrate and synthesizes Galβ1,4Xyl-Ser, which involved in at the linkage tetra saccharide region of chondroitin sulfate and heparan sulfate (7).
2) β1,4-N-Acetylgalactosaminyltransferase (β4GalNAc-T)
Four glycosyltransferases have been identified as β1,4-N-acetylgalactosaminyltransferases (β4GalNAc-T) in mammals including β4GalNAc-T1: GM2/GD2 synthase, β4GalNAc-T2: Sda synthase, β4GalNAc-T3 and β4GalNAc-T4: LacdiNAc (LDN, GalNAcβ1,4GlcNAc) synthase. β4GalNAc-T3 and β4GalNAc-T4 contain a β4GT motif, which is amino acid sequences conserved in β4Gal-T family too, except for β4GalNAc-T1 or β4GalNAc-T2. In this part, we briefly describe about LacdiNAc synthase, β4GalNAc-T3 and β4GalNAc-T4. LDN is a unique glycan structure originally discovered on N-glycans of glycoprotein hormones secreted from the pituitary gland and sulfated LDN have been reported to be necessary for glycoprotein hormones clearance from blood (8). LDN synthases, β4GalNAc-T3 and β4GalNAc-T4, were found to be expressed in many tissues such as brain, stomach, colon, kidney and others (9,10). LDN glycans have been reported on not only N-glycans but also O-glycans of various glycoproteins recently. Therefore, discovery of a novel biological function of the LDN structure is expected near future. |
| Figure & Legends |
Figure & Legends

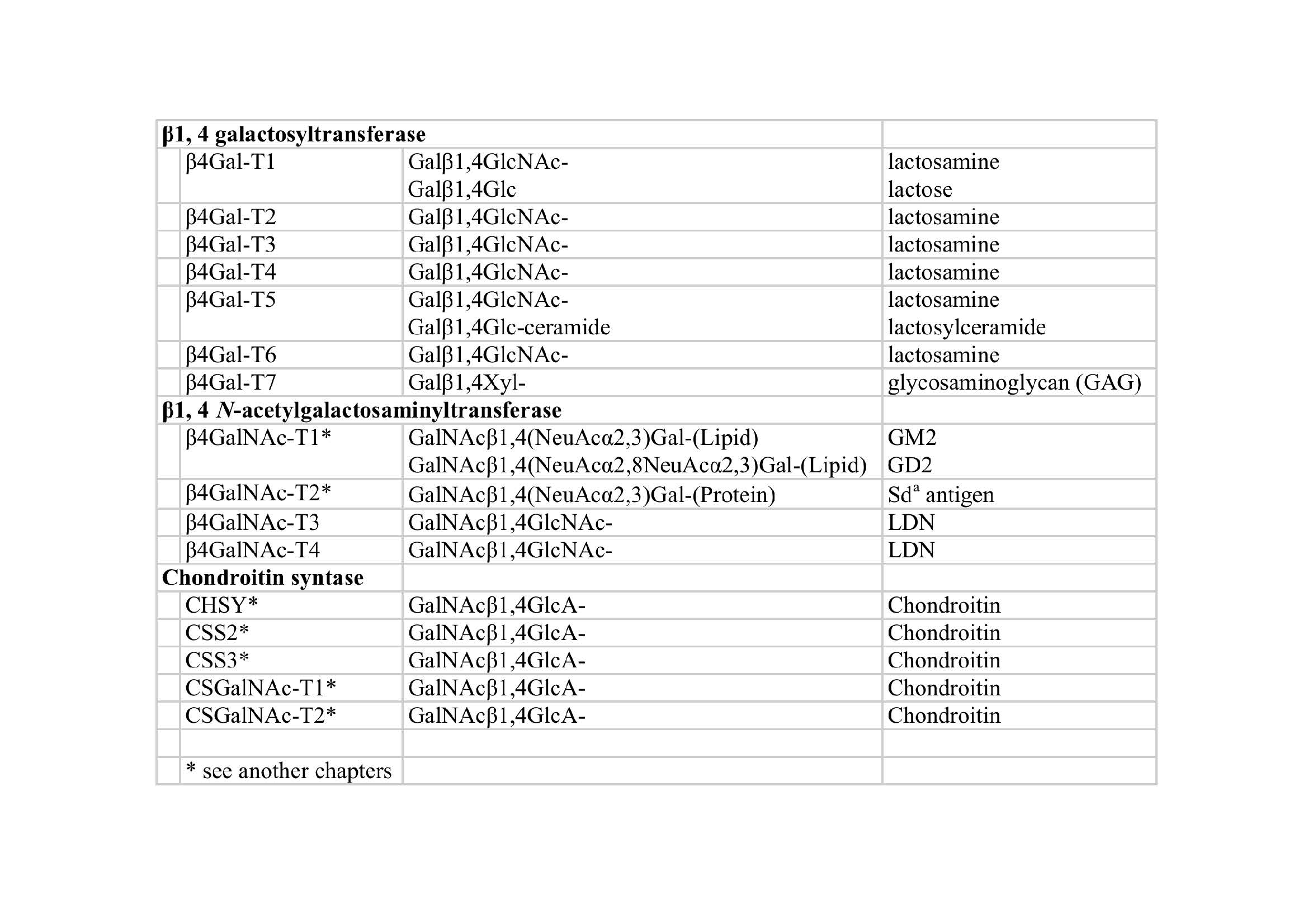
Fig. 1. Table; β1,4-Glycosyltransferases and their enzymatic activity |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2017-02-23 15:01:19 |
- Narimatsu, H., Sinha, S., Brew, K., Okayama, H., and Qasba, P. K. (1986) Cloning and sequencing of cDNA of bovine N-acetylglucosamine (β 1-4)galactosyltransferase. Proc Natl Acad Sci U S A 83, 4720–4724 [PMID : 3014508]
- Ito, H., Kameyama, A., Sato, T., Sukegawa, M., Ishida, H. K., and Narimatsu, H. (2007) Strategy for the fine characterization of glycosyltransferase specificity using isotopomer assembly. Nat Methods 4, 577–582 [PMID : 17529980]
- Seko, A., Dohmae, N., Takio, K., and Yamashita, K. (2003) β 1,4-galactosyltransferase (β4GalT)-IV is specific for GlcNAc 6-O-sulfate. β 4GalT-IV acts on keratan sulfate-related glycans and a precursor glycan of 6-sulfosialyl-Lewis X. J Biol Chem 278, 9150–9158 [PMID : 12511560]
- Nishie, T., Hikimochi, Y., Zama, K., Fukusumi, Y., Ito, M., Yokoyama, H., Naruse, C., and Asano, M. (2010) β4-galactosyltransferase-5 is a lactosylceramide synthase essential for mouse extra-embryonic development. Glycobiology 20, 1311–1322 [PMID : 20574042]
- Kumagai, T., Sato, T., Natsuka, S., Kobayashi, Y., Zhou, D., Shinkai, T., Hayakawa, S., and Furukawa, K. (2010) Involvement of murine β-1,4-galactosyltransferase V in lactosylceramide biosynthesis. Glycoconj J 27, 685–695 [PMID : 21057870]
- Takizawa, M., Nomura, T., Wakisaka, E., Yoshizuka, N., Aoki, J., Arai, H., Inoue, K., Hattori, M., and Matsuo, N. (1999) cDNA cloning and expression of human lactosylceramide synthase. Biochim Biophys Acta 1438, 301–304 [PMID : 10320813]
- Almeida, R., Levery, S. B., Mandel, U., Kresse, H., Schwientek, T., Bennett, E. P., and Clausen, H. (1999) Cloning and expression of a proteoglycan UDP-galactose:β-xylose β1,4-galactosyltransferase I. A seventh member of the human β4-galactosyltransferase gene family. J Biol Chem 274, 26165–26171 [PMID : 10473568]
- Fiete D, Srivastava V, Hindsgaul O, Baenziger JU. (1991) A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAc β 1,4GlcNAc β 1,2Man alpha that mediates rapid clearance of lutropin. Cell. 67, 1103–10 [PMID : 1662117]
- Sato, T., Gotoh, M., Kiyohara, K., Kameyama, A., Kubota, T., Kikuchi, N., Ishizuka, Y., Iwasaki, H., Togayachi, A., Kudo, T., Ohkura, T., Nakanishi, H., and Narimatsu, H. (2003) Molecular cloning and characterization of a Novel human β 1,4-N-acetylgalactosaminyltransferase, β 4GalNAc-T3, responsible for the synthesis of N,N'-diacetyllactosediamine, galNAc β 1-4GlcNAc. J Biol Chem 278, 47534–47544 [PMID : 12966086]
- Gotoh, M., Sato, T., Kiyohara, K., Kameyama, A., Kikuchi, N., Kwon, Y. D., Ishizuka, Y., Iwai, T., Nakanishi, H., and Narimatsu, H. (2004) Molecular cloning and characterization of β1,4-N-acetylgalactosaminyltransferases IV synthesizing N,N'-diacetyllactosediamine. FEBS Lett 562, 134–140 [PMID : 15044014]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Sato, Takashi,
Ito, Hiromi,
Narimatsu, Hisashi,
(2017). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.18,4,2024 .
How to Cite this Work in Website:
Sato, Takashi,
Ito, Hiromi,
Narimatsu, Hisashi,
(2017).
Enzyme assay of polypeptide N-acetylgalactosaminyltransferase, β1,3-glycosyltransferase, ang β1,4-glycosyltransferases. [3] β1,4-glycosyltransferases family.
Retrieved 18,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t242.
html source
Sato, Takashi,
Ito, Hiromi,
Narimatsu, Hisashi,
(2017).
<b>Enzyme assay of polypeptide <em>N-</em>acetylgalactosaminyltransferase, β1,3-glycosyltransferase, ang β1,4-glycosyltransferases. [3] β1,4-glycosyltransferases family</b>.
Retrieved 4 18,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t242" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t242</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|