Heparan sulfate (HS) proteoglycans on the cell surface and in the extracellular matrix regulate signal transduction of a variety of cell growth factors by interacting with those ligand molecules and their receptors. Here, the experiment to investigate regulation of the vascular endothelial growth factor (VEGF) activity with HS by the mitogenesis assay and the phosphorylation of VEGF receptor-2 (VEGFR-2) is shown for the examples. |
| Category | Matrices & cellular trafficking |
| Protocol Name | Regulation of growth factor activity by heparin/HSPG |
Authors
 |
Kimata, Koji
Research Complex for the Medicine Frontiers, Aichi Medical University
|
| KeyWords |
|
Reagents
 |
| ● |
Human umbilical vein endothelial cells (HUVEC) at passage numbers 4–7 (Clonetics, Walkersville, MD) |
| ● |
Recombinant murine VEGF165 (one of VEGF isoforms) (Diaclone, Cedex, France) |
| ● |
Recombinant murine VEGF121 (R & D Systems, Minneapolis, MN) |
| ● |
Rabbit polyclonal antibody against phosphorylated VEGFR-2 (Tyr 951) (Santa Cruz Biotechnology, Santa Cruz, CA) |
| ● |
Secondary horse radish peroxidase-conjugated goat anti-rabbit antibody (Organon Teknika Corp., Durham, NC) |
| ● |
Chemically modified heparin (CDSNS (completely desulfated and re-N-sulfated) heparin, 2ODS (2-O-desulfated) heparin, 6ODS (6-O-desulfated) heparin and PIO (periodate-oxidized) heparin) (Seikagaku Corp., Tokyo, Japan) |
| ● |
Heparin (Sigma-Aldrich, St. Louis, MO) |
| ● |
Heparitinase I (Flavobacterium heparinum, EC 4.2.2.8), heparitinase II (F. heparinum, no number assigned) and heparinase (F. heparinum, EC 4.2.2.7) (Seikagaku Corp.) |
| ● |
BrdU (5-bromo-2’-deoxyuridine) ELISA Kit (colorimetric) (Roche Applied Science, Penzberg, Germany) |
| ● |
Type I collagen-coated plate (96 wells) (BIOCOAT, BD, Franklin Lakes, NJ) |
| ● |
RPMI 1640 medium (Sigma-Aldrich) containing 0.5 % fetal bovine serum (FBS) and 0.2 mM glutamine |
| ● |
EGM-2 medium containing EBM-2MV SingleQuots® (Cambrex, East Rutherford, NJ) |
| ● |
Cell lysis buffer containing 10 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1% Triton X-100, 1.5 mMEDTA, 1 mM Na3PO4, 25 mM NaF, 1mM Na3VO4 and 1 tablet/10 mL of Protease Inhibitor Cocktail Tablets (complete, mini, Roche Diagnostics GmbH, Manheim, Germany) |
| ● |
QuantiPro BCA assay kit (Sigma-Aldrich) |
| ● |
10% skim milk containing PBS for the blotting of proteins |
| ● |
1% skim milk containing PBS for the dilution of the primary antibody for the immuno-staining of the blotted proteins |
| ● |
Enhancer solution of chemiluminescence (Western Lightning Plus, PerkinElmer, Waltham, MA) |
|
Instruments
 |
| ● |
VERSAmax for measuring the absorbance (Molecular Devices, Sunnyvale, CA) |
| ● |
Image Gauge V3.45 for the determination of the fluorescent band density (Fujifilm, Tokyo, Japan) |
|
| Methods |
|
1. |
Effect of heparin and modified heparins on VEGF-induced mitogenic responses (see Note 1) |
| 1) |
HUVEC were seeded at a density of 2 × 103 cells/well on a type I collagen-coated plate (96 wells) in 100 μL of RPMI 1640 medium containing 0.5 % FBS and 0.2 mM glutamine. |
Comment 0
|

|
| 2) |
After 1 h, VEGF165 or VEGF121 (final concentration: 10 ng/mL) was added with various concentrations of heparin or modified heparin. |
Comment 0
|

|
| 3) |
After 24 h, BrdU at the final concentration (10 mM) recommended by the manufacturer in the ELISA kit was also added and the cells were further incubated for 24 h. |
Comment 0
|

|
| 4) |
Remove the culture medium, and the cells were fixed in 10% formalin containing PBS. |
Comment 0
|

|
| 5) |
Apply the colorimetric BrdU ELISA kit where peroxidase-conjugated anti-BrdU antibody bound to BrdU incorporated in the newly synthesized, cellular DNA. |
Comment 0
|

|
| 6) |
The immune complexes were detected by the subsequent substrate reaction of peroxidase in the above kit. |
Comment 0
|

|
| 7) |
Quantify the complexes by measuring the absorbance using VERSAmax. |
Comment 0
|

|
| 8) |
Repeat the above experiments independently three times for the statistical analyses using Student’s t test. |
Comment 0
|
|
|
|
2. |
Effects of heparin on VEGF165 activity (see Note 2) |
| 1) |
HUVEC were seeded at 1.9 × 105 cells/well on a 12-well tissue culture plate in 1 mL EGM-2 medium containing EBM-2MV SingleQuots®. |
Comment 0
|

|
| 2) |
The cells were serum-starved overnight in EGM-2 medium. |
Comment 0
|

|
| 3) |
The cells were then stimulated with 10 ng/mL VEGF165 or VEGF121 in the presence or absence of heparin for 5 min at 37oC. |
Comment 0
|

|
| 4) |
The cells were lysed in 500 mL/well of cell lysis buffer containing 10 mM Tris-HCl, pH 7.4, 140 mM NaCl, 1% Triton X-100, 1.5 mM EDTA, 1 mM Na3PO4, 25 mM NaF, 1mM Na3VO4 and 1 tablet/10 mL of Protease Inhibitor Cocktail Tablets. |
Comment 0
|

|
| 5) |
Cells in lysates were sit at −80°C overnight, collected into centrifugation tubes with a plastic scraper, and gently stirred. The lysates were then clarified by centrifugation at 10,000 rpm for 30 min. |
Comment 0
|

|
| 6) |
Measure protein concentrations using the QuantiPro BCA assay kit. |
Comment 0
|

|
| 7) |
For immunoblotting, cell lysates (30 μg of protein from each sample) were subjected to 7.0% (w/v) SDS-PAGE and transferred to a PVDF membrane as usual. |
Comment 0
|

|
| 8) |
The blots were incubated with 10% skim milk containing PBS and probed with the primary antibody diluted in 1% skim milk containing PBS. |
Comment 0
|

|
| 9) |
The signal was visualized using horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence. |
Comment 0
|

|
| 10) |
Band density was determined using Image Gauge. |
Comment 0
|
|
|
|
3. |
Involvement of cell surface heparan sulfate in the VEGF165-induced phosphorylation of VEGFR-2 (see Note 3) |
| 1) |
HUVEC were seeded and then serum-starved overnight as described above. |
Comment 0
|

|
| 2) |
The cells/well were digested with 10 mU of heparitinase I, 5 mU of heparitinase II and 10 mU of heparinase (HSase-mixture) at 37°C for 15 min (1 U is defined as the activity to be able to digest 1 mmole of substrates per 1 min). |
Comment 0
|

|
| 3) |
Wells were subjected to several washes with EGM-2 medium for the complete removal of enzymes and released glycosaminoglycan fragments. |
Comment 0
|

|
| 4) |
The cells were then stimulated and subjected to the analysis for the phosphorylation as described above. |
Comment 0
|
|
|
| Notes | 1. The VEGF-induced incorporation of BrdU into HUVEC was examined by culturing the cells in the presence of 10 ng/mL VEGF165 or VEGF121 with various concentrations of heparin or modified heparins.
The VEGF165-induced proliferation was significantly enhanced dose-dependently only by heparin (heparin at 1 and 10 μg/mLstimulated proliferation 1.4- and 1.8-fold, respectively, compared to no added heparin) (Fig. 1A). None of modified heparins affected the proliferation of HUVEC. In contrast, the VEGF121-dependent incorporation of BrdU was not enhanced by heparin or by any modified heparins at any concentrations tested (Fig. 1B). The results indicate that both 2-O- and 6-O-sulfate residues in heparin are necessary for the enhancement of VEGF165-induced proliferation by heparin, but heparin is not involved in VEGF121-dependent proliferation.
2. By Western blot analysis using a specific antibody for phospho-VEGFR-2, the tyrosine phosphorylation of VEGFR-2 was examined after the enhancement of VEGF165 activity by heparin, since VEGF is known to stimulate the autophosphorylation of VEGFR-2.
Treatment with VEGF165 alone for 5 min induced VEGFR-2 phosphorylation (Fig. 2A lanes 1 and 4). In the presence of heparin, the VEGF165-induced phosphorylation of VEGFR-2 increased 1.7-fold (Figs. 2A lanes 4–6, 2B gray bars). Heparin alone did not substantially affect VEGFR-2 phosphorylation (Fig. 2A lanes 1–3). The VEGF121-induced phosphorylation of VEGFR-2 did not increase in the presence of heparin (Figs. 2C and D), suggesting that the heparin-enhanced phosphorylation of VEGFR-2 occurred through the interaction with VEGF165, but not with VEGF121.
3. The involvement of cell surface HS in the VEGF165-induced phosphorylation of VEGFR-2 was examined by the VEGF165-induced receptor phosphorylation in HUVEC with and without the heparitinase mixture-treatment. Cells were treated with or without HSase mixture, washed (the digestion resulted in the removal of more than 95% of cell surface/ECM HS), and then stimulated with VEGF165 in the absence or presence of heparin for 5 min at 37°C. VEGF165-induced phosphorylation of VEGFR-2 was reduced to 75% by HSase mixture treatment (Figs. 3A lanes 1 and 2, 3B). In contrast, digestion with HSase mixture did not affect VEGF121-induced phosphorylation of VEGFR-2 (Figs. 3C and D). The reduction of VEGF165-induced phosphorylation by digestion with HSase mixture was rescued by the addition of heparin (Figs. 3A lanes 3–6, 3B). It is known that VEGFR-2 induces cell proliferation through activation of the classical extracellular regulated kinase (ERK) pathway, leading to gene transcription. As expected, phosphorylation of ERK1/2 increased 2.5-fold, 5 min after stimulation by VEGF165 (Figs. 3E lanes 1 and 2, 3F); this phosphorylation was markedly decreased (to 25%) by HSase-mixture digestion, and restored by exogenous heparin (Figs. 3E lanes 3–5, 3F). The results suggest that cell-surface HS regulated VEGFR-2 activation mediated by heparin-binding VEGF. |
| Discussion | The experiments here demonstrated enhancement effects of heparin not only on the VEGF165-induced proliferation of HUVEC but also on the VEGF-induced phosphorylation of VEGFR-2, which suggests that heparin facilitates to form a signaling complex with VEGF165 and VEGFR-2 (see Fig. 4). The digestion of the cells with HSase mixture resulted in the reduction of the VEGF165-induced phosphorylation of VEGFR-2 to 75% with significance (Fig. 3A), but the reduction was rescued by the addition of heparin (Figs.3A lanes 3–6, 3B), suggesting that the heparin-like domain of cell surface HS positively regulated VEGF165 activity. Since the addition of heparin and digestion with HSase mixture did not affect VEGF121-induced phosphorylation of VEGFR-2 (Fig. 1B & Figs. 3C and D, respectively), the cell surface HS was not essential for VEGFR-2 phosphorylation. However, mice expressing the VEGF121 isoform exclusively via the Cre/loxP-mediated removal of exons 6 and 7, which encode the heparin-binding region, had impaired postnatal myocardial angiogenesis, and ultimately died of cardiac failur (Careliet, P., et al. 1999. Nat. Med., 5: 495–502). VEGF121 alone was insufficient for normal angiogenesis, suggesting the important regulatory function of the interactions between the heparin-like domain of cell surface HS and the heparin binding domains of VEGF isoforms.
Neither 2ODS heparin nor 6ODS heparin enhanced the VEGF165-dependent proliferation (Fig.1A). Considering that heparin is known to interact with receptors for VE GF165, VEGFR-2 and neuropilin (NRP)-1, 2ODS heparin and 6ODS heparin may lack the structure required for binding to those VEGF165 receptors, and the heparin-like structural domain of HS may greatly contribute to the enhanced formation of a signaling complex between VEGF165 and its receptors.
Here we propose an action mechanism of VEGF165 activity enhancement by heparin considering the reported roles of NRP-1, as shown in Fig. 4. In the absence of heparin, both VEGF165 binding to VEGFR-2 and VEGF165-dependent phosphorylation of VEGFR-2 are regulated by cell surface HSPG via binding to the heparin-like domain. In the presence of heparin, VEGF165, VEGFR-2 and NRP-1 form a complex through the intermediary molecule of heparin. In consequence, VEGF165-induced signaling processes, such as the phosphorylation of VEGFR-2, are increased by heparin. This mechanism is also suggested by the following results reported by others: Heparin enhances VEGF165 binding to VEGFR, the affinity of VEGF165 for NRP-1 is also enhanced by the addition of heparin, NRP-1 in turn enhances VEGF165 binding to VEGFR-2 and its bioactivity, heparin induced the binding of NRP-1 to VEGF165, and in consequence, heparin may regulate VEGF165 activity selectively via NRP-1. We should also consider another mechanism of VEGF165 activity enhancement by heparin: VEGF165 stability was increased by complexing with heparin. |
| Figure & Legends |
Figure & Legends 
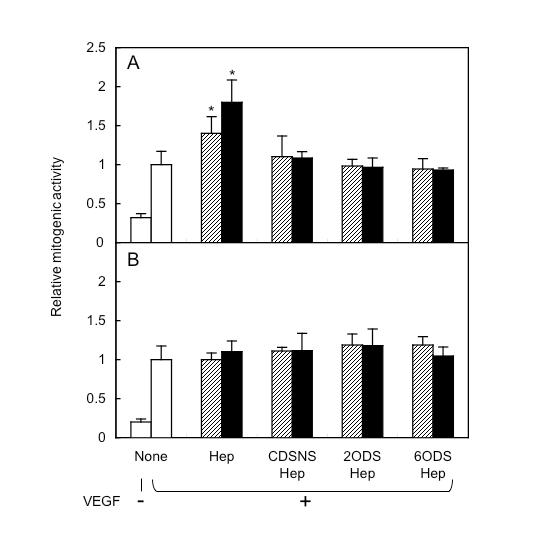
Fig. 1. Effect of heparin and modified heparins on VEGF-induced proliferation of HUVEC
HUVEC were seeded on a 96-well plate. The medium contained 10 ng/mL VEGF165 (A) or VEGF121 (B) in the absence (open bars), or presence of 1 (striped bars) or 10 μg/mL (closed bars) heparin or modified heparins. After 24 h of incubation, BrdU was added and the cells reincubated for an additional 24 h. Each bar represents the relative mitogenic activity (ratio of incorporation to that in VEGF alone). Data are the averages of triplicate measurements with standard deviation. Asterisks indicate significant differences from VEGF alone as determined by t test (p<0.05). The same results were obtained from two independent experiments. (Redrawn, with permission, from Ashikari-Hada S., et al. 2005. J. Biol. Chem. 280: 31508–15)
This figure was originally published in J Biol Chem. Ashikari-Hada S. et al. "Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins" 2005, 280(36):31508–15. © the American Society for Biochemistry and Molecular Biology.

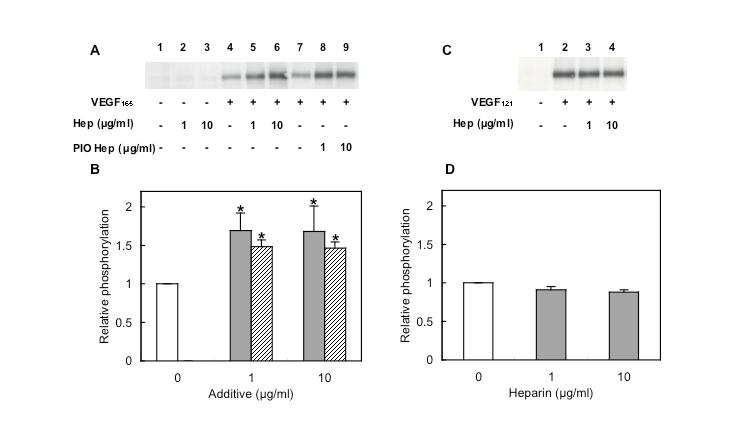
Fig. 2. Increase of VEGF165-induced phosphorylation of VEGFR-2 by the addition of heparin
HUVEC were stimulated with 10 ng/mL VEGF165 (A) or VEGF121 (C) in the absence (open bars) or presence of heparin (gray bars) or PIO heparin (striped bars) for 5 min at 37°C. Samples were subjected to gel electrophoresis, with the subsequent transfer of membranes. Phospho-VEGFR-2 was stained with its specific antibody. VEGF165-dependent (B) and VEGF121-dependent (D) VEGFR-2 phosphorylation were measured using Image Gauge. Each bar represents the mean value of four (B) and three (D) independent experiments. Asterisks indicate significant differences from VEGF alone as determined by t test (p<0.05). (Redrawn, with permission, from Ashikari-Hada S. et al. 2005)
This figure was originally published in J Biol Chem. Ashikari-Hada S. et al. "Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins" 2005, 280(36):31508–15. © the American Society for Biochemistry and Molecular Biology.

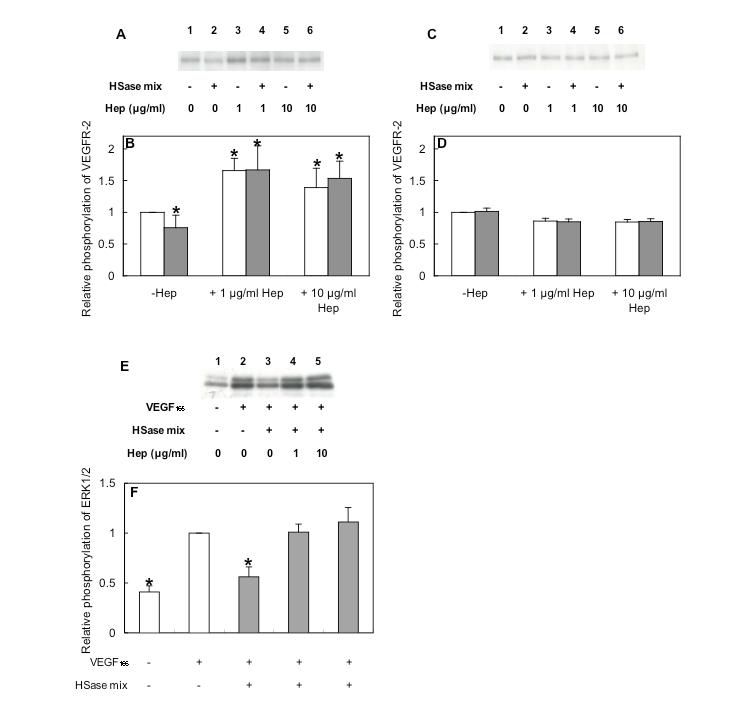
Fig. 3. Restoration of VEGF165-induced phosphorylation in heparinase-treated HUVEC by heparin
HUVEC were digested with or without HSase mixture, and then stimulated with 10 ng/mL VEGF165 (A and E) or VEGF121 (C) in the presence or absence of heparin for 5 min at 37°C. Samples were subjected to gel electrophoresis, and then toWestern blotting using anti-phospho-VEGFR-2 antibody (A and C) or anti-phospho-ERK1/2 antibody (E). VEGFR-2 phosphorylation (B and D) and ERK1/2 phosphorylation (F) were quantified, and each bar (B and F) indicates the relative phosphorylation to that of VEGF alone and the average of three independent experiments and standard deviation. Asterisks indicate significant differences from VEGF alone as determined by t test (p<0.05). (Redrawn, with permission, from Ashikari-Hada S. et al. 2005)
This figure was originally published in J Biol Chem. Ashikari-Hada S. et al. "Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins" 2005, 280(36):31508–15. © the American Society for Biochemistry and Molecular Biology.

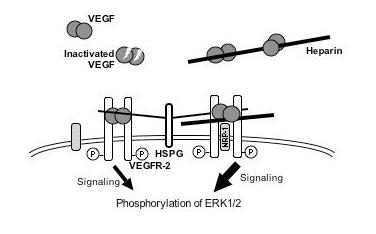
Fig. 4. Postulated model of the signaling by the interactions of VEGF165, VEGFR-2 and heparin
The VEGF165-dependent phosphorylation of VEGFR-2 was increased by interaction with HS proteoglycans on the cell surface. Added heparin also regulates VEGF165 activity by participating in the formation of a complex of VEGF, VEGFR-2 and NRP-1. Further, it is also involved in VEGF165 stability. (Redrawn, with permission, from Ashikari-Hada S. et al. 2005)
This figure was originally published in J Biol Chem. Ashikari-Hada S. et al. "Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. Comparison of the effects of heparin and modified heparins" 2005, 280(36):31508–15. © the American Society for Biochemistry and Molecular Biology. |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2014-06-05 10:20:25 |
- Habuchi, H., Tanaka, M., Habuchi, O., Yoshida, K., Suzuki, H., Ban, K., and Kimata, K. (2000) The occurrence of three isoforms of heparan sulfate 6-O-sulfotransferase having different specificities for hexuronic acid adjacent to the targeted N-sulfoglucosamine. J Biol Chem. 275, 2859−2868. [PMID : 10644753]
- Ashikari-Hada, S., Habuchi, H., Kariya, Y., Itoh, N., Reddi, A. H., and Kimata, K. (2004) Characterization of growth factor-binding structures in heparin/heparan sulfate using an octasaccharide library. J Biol Chem. 279, 12346−12354. [PMID : 14707131]
- Ashikari-Hada, S., Habuchi, H., Kariya, Y., and Kimata, K. (2005) Heparin regulates vascular endothelial growth factor165-dependent mitogenic activity, tube formation, and its receptor phosphorylation of human endothelial cells. J Biol Chem. 280, 31508–15. [PMID : 16027124]
- Sugaya, N., Habuchi, H., Nagai, N., Ashikari-Hada, S., and Kimata, K. (2008) 6-O-sulfation of heparan sulfate differentially regulates various fibroblast growth factor-dependent signalings in culture. J Biol Chem. 283, 10366–76. [PMID : 18281280]
- Ashikari-Hada, S., Habuchi, H., Sugaya, N., Kobayashi, T., and Kimata, K. (2009) Specific inhibition of FGF-2 signaling with 2-O-sulfated octasaccharides of heparan sulfate. Glycobiology 19, 644–54. [PMID : 19254961]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Kimata, Koji,
(2014). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.19,4,2024 .
How to Cite this Work in Website:
Kimata, Koji,
(2014).
Regulation of growth factor activity by heparin/HSPG.
Retrieved 19,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t24.
html source
Kimata, Koji,
(2014).
<b>Regulation of growth factor activity by heparin/HSPG</b>.
Retrieved 4 19,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t24" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t24</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|