For atomic-level structural analyses such as X-ray crystallography and NMR spectroscopy, the large amount (1–10 mg) of highly purified protein is often required. Bacterial expression system is the most powerful method to produce a large amount of recombinant proteins, however it is not effective to produce originally glycosylated proteins due to the lack of glycosylation and inefficient disulfide bond formation. Alternatively, mammalian expression system using several cell lines of which glycosyltransferase are mutated is a useful platform to produce glycoproteins with disulfide bonds. In this section, we introduce a protocol of mammalian expression system for glycoprotein structural analysis. |
| Category | Isolation & structural analysis of glycans |
| Protocol Name | Preparation of glycoprotein for structural analysis |
Authors
 |
Nagae, Masamichi
Structural Glycobiology Team, RIKEN Global Research Cluster
Yamaguchi, Yoshiki
*
Structural Glycobiology Team, RIKEN
*To whom correspondence should be addressed.
|
| KeyWords |
|
Reagents
 |
| ● |
MEM alpha (Invitrogen/Life Technologies, Carlsbad, CA, 12714-010) |
| ● |
DMEM + L-Glu (Gibco/Life Technologies, Carlsbad, CA, 11965) |
| ● |
Fetal bovine serum (FBS ) (PAA/GE Healthcare, Little Chalfont, UK, A15-751) |
| ● |
MEM Non-essential amino acid solution (NEAA) 100× (Sigma-Aldrich, St. Louis, MO, M7145) |
| ● |
100mM Sodium Pyruvate solution (Gibco/Life Technologies, 11360) |
| ● |
Penicillin Streptmycin mixed (Pen Strept) solution (Sigma-Aldrich, P4458) |
| ● |
G418 sulfate (Calbiochem, San Diego, CA, 345810) |
| ● |
Puromycin (Sigma-Aldrich, P7255) |
| ● |
Polyethylenimine liner (PEI) (Polysciences, Inc., Warrington, PA, 23966) |
| ● |
Opti-MEM (1x) (Gibco/Life Technologies, 31985) |
|
Instruments
 |
| ● |
BICELL (Nihon Freezer Co., Ltd., Tokyo, Japan) |
| ● |
Roller Bottle (Corning, NY, 431135) |
| ● |
Forma Series II CO2 incubator (Thermo Fisher Scientific Inc., Waltham, MA) |
| ● |
Cell-production Roller apparatus (BELLCO) |
| ● |
|
|
| Methods |
|
1. |
|
| 1) |
Prepare DMEM complete medium for HEK293 GnTI-/- cell (Reeves PJ et al., 2002). Mix DMEM 500 mL with 50 mL FBS, 5 mL NEAA, 5 mL pyruvate and 2.75mL Pen Strept. |
Comment 0
|

|
| 2) |
Prepare MEM alpha complete medium for CHO-Lec3.2.8.1 cell (Stanley 1989). Mix MEM alpha 500mL with 27.5mL FCS, 5mL NEAA, 5 mL pyruvate and 2.75mL Pen Strept. |
Comment 0
|

|
| 3) |
Prepare G418 stock solution (final conc. 100 mg/mL). Dissolve 1g G418 with 10 mL distilled water and filtrate for sterilization. |
Comment 0
|

|
| 4) |
Prepare puromycin stock solution (final conc. 5 mg/mL). Dissolve 50 mg puromycin with 10 mL DMEM and filtrate for sterilization. |
Comment 0
|

|
| 5) |
Prepare PEI stock solution (final conc. 1mg/mL). Dissolve 50 mg PEI with 50 mL distilled water. Warm solution at 55°C for ~30 min to dissolve it completely. Adjust pH to ~7 with 1 N HCl. Filtrate for sterilization. |
Comment 0
|

|
| 6) |
Prepare freezing medium. Mix DMEM (or MEM alpha) with 20%(v/v) FCS and 10%(v/v) DMSO |
Comment 0
|
|
|
|
2. |
Transient (or stable) expression |
| 1) |
Prepare plasmid harboring target gene. The target protein can be expressed as fusion protein with affinity tag. Several constructs with different expression regions will be required. |
Comment 0
|

|
| 2) |
Prepare the confluent HEK293 GnTI-/- or CHO-Lec3.2.8.1. cells with 12-well plastic plate. Mix plasmid with PEI solution and incubate at room temperature. Add mixed solution to culture medium. |
Comment 0
|

|
| 3) |
After several days, collect culture medium (~1 mL/well). Perform pull down assay using affinity resin (Ni-NTA resin) or immunoprecipitation assay using anti-affinity tag antibody. Apply the samples to SDS-PAGE and estimate the expression levels of each sample. |
Comment 0
|

|
| 4) |
Establish stable cell lines which express high-yield target proteins. |
Comment 0
|
|
|
|
3. |
|
| 1) |
Prepare the culture medium by the addition of antibiotics for selection marker (final conc. 0.5 mg/mL G418 and/or 2 μg/mL puromycin). |
Comment 0
|

|
| 2) |
Expand stable cell in T-flask until it reaches ~108 cells. |
Comment 0
|

|
| 3) |
Inoculate roller bottle and incubate for one week. |
Comment 0
|
|
|
|
4. |
|
| 1) |
Suspend cells in 10-cm dish with freezing medium. |
Comment 0
|

|
| 2) |
Put 1-mL suspension (final conc. 107 cell/mL) into 1 vial (Cryo Tube). |
Comment 0
|

|
| 3) |
Put vials into BICELL and store −80°C freezer for ~1 day. Store the vials in liquid nitrogen tank. |
Comment 0
|
|
|
| Notes | Since secreted glycoproteins have signal sequence at the N-terminus, affinity tag such as His-tag is usually attached at the C-terminus. The affinity tag should be removable by specific proteases.
The glycans attached on protein occasionally prevent crystallization. However, the mutation of glycosylation site might affect the solubility of target proteins. For removal of the surface glycans, deglycosylation using endoglycosidase is effective to improve the quality of crystals (Chang et al., 2007; Nakata et al., 2011). |
| Figure & Legends |
Figure & Legends

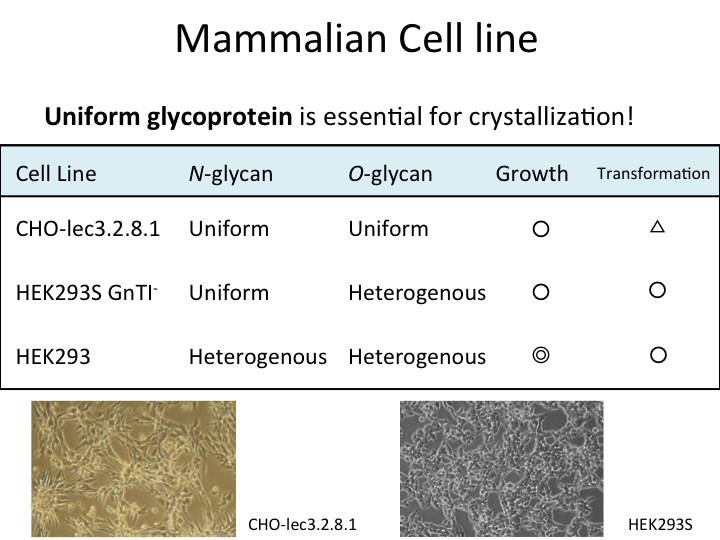
Fig. 1. Comparison of three representative mammalian cell lines which are used for structural analyses |
| Copyrights |
 Attribution-Non-Commercial Share Alike Attribution-Non-Commercial Share Alike
This work is released underCreative Commons licenses
|
| Date of registration:2015-02-13 11:33:21 |
- Reeves, P.J., Callewaert, N., Contreras, R., and Khorana, H.G., (2002) Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc.Natl.Acad.Sci. USA 99, 13419–24 [PMID : 12370423]
- Stanley, P. (1989) Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol. Cell Biol. 9, 377–383 [PID : 2710109]
- Chang, V.T., Crispin, M., Aricescu, A.R., Harvey, D.J., Nettleship, J.E., Fennelly, J.A., Yu, C., Boles, K.S., Evans, E.J., Stuart, D.I., Dwek, R.A., Jones, E.Y., Owens, R.J., and Davis, S.J., (2007) Glycoprotein structural genomics: solving the glycosylation problem. Structure 15, 267–73 [PMID : 17355862]
- Nakata, Z., Nagae, M., Yasui, N., Bujo, H., Nogi, T., and Takagi, J. (2011) Crystallization and preliminary crystallographic analysis of human LR11 Vps10p domain. Acta Crystallogr Sect F Struct Biol Cryst Commun. 67 129–132 [PMID : 21206043]
|
This work is licensed under Creative Commons Attribution-Non-Commercial Share Alike. Please include the following citation
How to Cite this Work in an article:
Nagae, Masamichi,
Yamaguchi, Yoshiki,
(2015). GlycoPOD https://jcggdb.jp/GlycoPOD.
Web.20,4,2024 .
How to Cite this Work in Website:
Nagae, Masamichi,
Yamaguchi, Yoshiki,
(2015).
Preparation of glycoprotein for structural analysis.
Retrieved 20,4,2024 ,
from https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t236.
html source
Nagae, Masamichi,
Yamaguchi, Yoshiki,
(2015).
<b>Preparation of glycoprotein for structural analysis</b>.
Retrieved 4 20,2024 ,
from <a href="https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t236" target="_blank">https://jcggdb.jp/GlycoPOD/protocolShow.action?nodeId=t236</a>.
Including references that appeared in the References tab in your work is
much appreciated.
For those who wish to reuse the figures/tables, please contact JCGGDB
management office (jcggdb-ml@aist.go.jp).
|
|