Peroxidase staining method is widely used and a powerful technique to detect localization of proteins on cells and tissues, and has an advantage of long-time preservation of slides. This protocol is for a detection of specific carbohydrate structures with a peroxidase lectin by using a horseradish peroxidase (HRPO)-based detection system. The procedure relies on proper fixation of cells to retain cellular distribution of antigen and to preserve cellular morphology. |
| Category | Sugar binding proteins |
| Protocol Name | Histochemical analysis of cell surface glycans by peroxidase labeled lectin |
Authors
 |
Nonaka, Motohiro
Laboratory for Drug Discovery, Biotechnology Research Institute for Drug Discovery, Department of Life Science and Biotechnology, National Institute of Advanced Industrial Science and Technology (AIST)
|
Reagents
 |
| ● |
|
| ● |
|
| ● |
10% (v/v) neutral buffered formalin or 4% paraformaldehyde in PBS, pH 7.4 |
| ● |
|
| ● |
|
| ● |
|
| ● |
0.01M Citrate buffer, pH 6.0 |
| ● |
Blocking solution (1% BSA in PBS) |
| ● |
HRPO-conjugated avidin-biotin complex (ABC) |
| ● |
|
| ● |
|
| ● |
|
|
Instruments
 |
| ● |
|
| ● |
|
| ● |
|
| ● |
Poly-L-lysine-coated slides |
| ● |
|
| ● |
|
| ● |
|
| ● |
|
| ● |
|
|
| Methods |
|
1. |
Paraffin embedding of the tissues |
| 1) |
Immerse the tissue fragment in 10% neutral buffered formalin or 4% paraformaldehyde in PBS. |
Comment 0
|

|
| 3) |
Immerse the tissue in 75% ethanol for 15 min. |
Comment 0
|

|
| 4) |
Immerse the tissue in 95% ethanol for 15 min and then for 20 min. |
Comment 0
|

|
| 5) |
Immerse the tissue in 100% ethanol three times for 20 min each. |
Comment 0
|

|
| 6) |
Immerse the tissue in xylene three times for 20 min each. |
Comment 0
|

|
| 8) |
Section 5–8 μm thick, and place the sections on poly-L-lysine-coated slides. |
Comment 0
|

|
| 9) |
Incubate the slides in a dry oven at 60°C for 1 h. |
Comment 1
|
|
|
|
2. |
Deparaffinaization and hydration of paraffin-embedded section |
| 1) |
Immerse the slides in a Coplin jar containing xylene five times for 4 min each. |
Comment 0
|

|
| 2) |
Immerse the tissue in 100% ethanol twice for 3 min each. |
Comment 0
|

|
| 3) |
Immerse the tissue in 95% ethanol twice for 3 min each. |
Comment 0
|

|
| 4) |
Immerse the tissue in 75% ethanol twice for 3 min each. |
Comment 0
|

|
| 5) |
Immerse slides in tap water for 5 min. |
Comment 0
|
|
|
|
3. |
|
| 1) |
Immerse slides into citrate buffer (0.01M, pH6.0). |
Comment 0
|

|
|
|
|
4. |
Blocking of the endogenous peroxidase |
| 1) |
Incubate the slide in 0.3% H2O2/methanol for more than 20 min. |
Comment 1
|

|
|
|
|
5. |
|
| 1) |
Using a pen containing water-repellant wax, outline the tissue sections on the glass slide. |
Comment 0
|

|
| 2) |
Place the slide in a staining chamber. |
Comment 0
|

|
| 3) |
Add blocking solution to the slides. |
Comment 0
|

|
| 5) |
Remove as much of the solution as possible by tilting the slides. |
Comment 0
|

|
| 6) |
Apply biotinylated lectin in sufficient quantity to cover the tissue. |
Comment 1
|

|
| 7) |
Incubate at room temperature for 1 h in the dark. |
Comment 0
|

|
| 8) |
Wash the slides three times for 5 min each. |
Comment 0
|
|
|
|
6. |
Detection of bound lectin |
| 1) |
Incubate the slide for 20 min in HRPO-conjugated avidin-biotin complex (ABC). |
Comment 0
|

|
| 2) |
Wash the slides three times for 5 min each. |
Comment 0
|

|
| 3) |
Add AEC substrate solution to the slide and incubate for 10 min. |
Comment 0
|

|
| 4) |
Transfer the slide to a Coplin jar. |
Comment 0
|

|
| 5) |
Wash for 10 min in running tap water. |
Comment 0
|
|
|
|
7. |
|
| 1) |
Place 1 drop of mounting medium onto slide. |
Comment 0
|

|
| 3) |
Gently blot mounted coverslip with paper towel. |
Comment 0
|

|
| 4) |
Seal edge of coverslip onto slide by painting the edge with a rim of nail polish and let dry. |
Comment 0
|

|
| 5) |
View specimen on light microscope. |
Comment 1
|
|
|
| Notes | Fixed, washed coverslips can be stored in PBS at 4°C for several days before staining. Incubation with lectin can be extended to overnight at 4°C if necessary. Tissues can be counterstained with hematoxylin if necessary. If background staining with lectin is too high, the lectin solution may be diluted further, wash for longer time, or try higher concentration of BSA in blocking solution. If specific staining is observed, but it is very faint, it is possible to increase the concentration of the lectin solution. |
| Figure & Legends |
Figure & Legends 
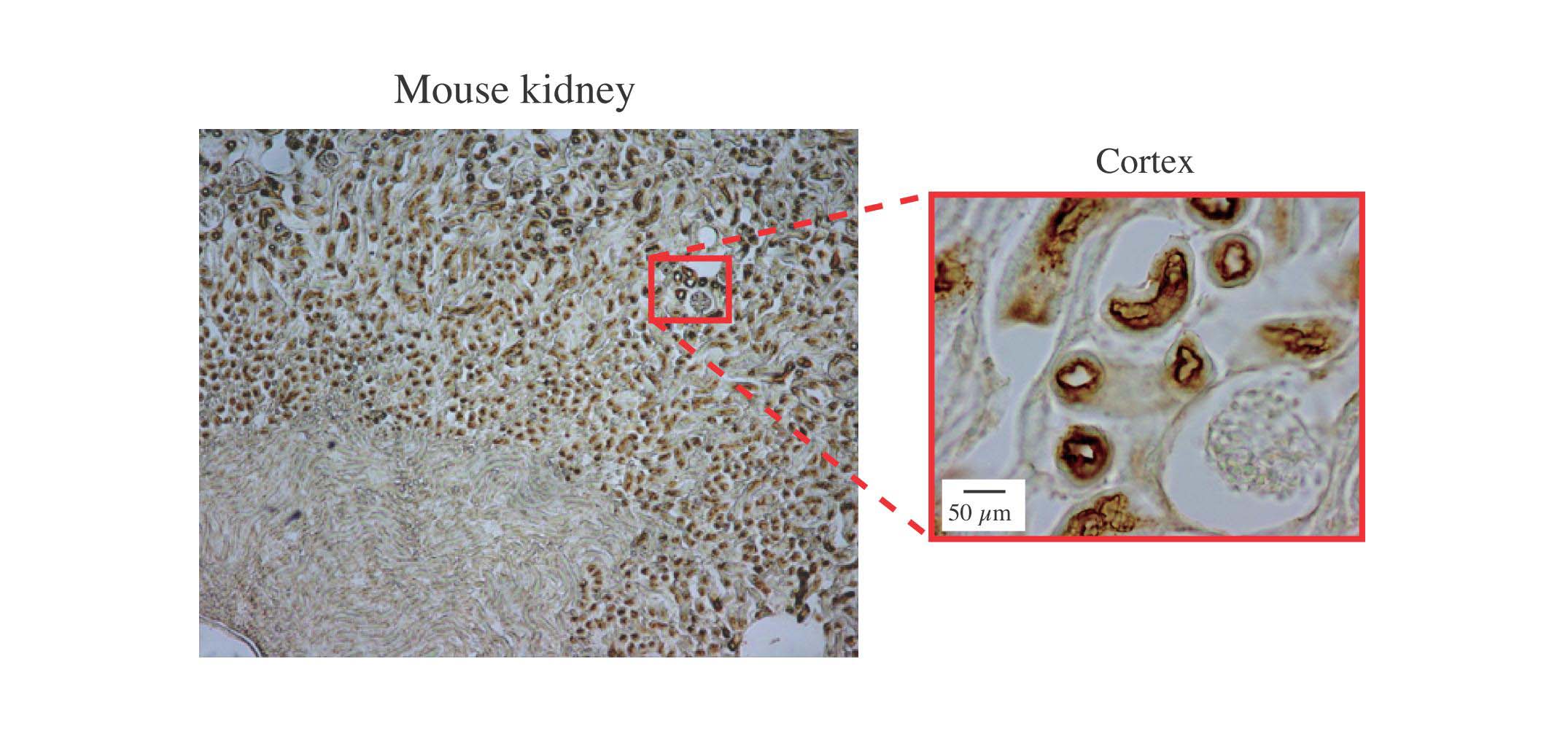
Fig. 1. Histochemical analysis of human kidney stained by peroxidase labeled MBP
The section of mouse kidney was stained with biotin conjugated human MBP followed by HRP labeled avidin. The section was also counterstained with hematoxylin. Magnification of the cortex indicates brush border membrane staining in proximal renal tubules and the lack of staining in renal corpuscles.
This figure was originally published in J Immunol. Hirano M, Ma BY. et al. "Mannan-binding protein blocks the activation of metalloproteases meprin alpha and beta" 2005, 175(5):3177–85. © The American Association of Immunologists, Inc.
|
| Copyrights |
Copyright 2005. The American Association of Immunologists, Inc. for Fig.1 in Figure & Legends
Copyright 2010. Ritsumeikan University, JCGGDB & AIST. for the rest of the contents |
| Date of registration:2015-02-25 11:30:28 |
- Current protocols in immunology (Vol. 5), John Wiley & Sons, Inc.
- Shin-sensyokuhou (Medical Technology Sup.), Ishiyaku Publishers, Inc.
|
|
For those who wish to reuse the work, please contact JCGGDB management office (jcggdb-ml@aist.go.jp).
|
|